Synthesis, Spectroscopic, and Biological Studies on New Zirconium(IV) Porphyrins with Axial Ligand
- PMID: 24106455
- PMCID: PMC3782819
- DOI: 10.1155/2013/903616
Synthesis, Spectroscopic, and Biological Studies on New Zirconium(IV) Porphyrins with Axial Ligand
Abstract
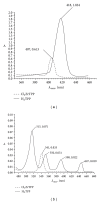
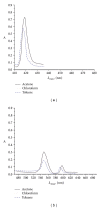
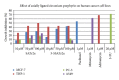
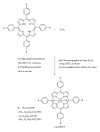
A series of parasubstituted tetraphenylporphyrin zirconium(IV) salicylate complexes (SA/5-SSAZr(IV)RTPP, R = p-H, p-CH3, p-NO2, p-Cl, SA = salicylate, and 5-SSA = 5-sulfosalicylate) have been synthesized, and the spectral properties of free base porphyrins, their corresponding metallated, and axially ligated zirconium(IV) porphyrin compounds were compared with each other. A detailed analysis of ultraviolet-visible (UV-vis), proton nulcear magnetic resonance ((1)H NMR) spectroscopy, infrared (IR) spectroscopy, and elemental analysis suggested the transformation from free base porphyrins to zirconium(IV) porphyrins. The ability of the metal in this complex for extra coordination of solvent molecules was confirmed by ESI-MS spectra. Besides the fluorescence, cyclic voltammetry, and thermogravimetric studies, the complexes were also screened for antimicrobial and anticancer activities. Among all the complexes, 5-SSAZr(p-NO2TPP) shows high antibacterial activity.
Figures





Similar articles
-
Synthesis and Spectroscopic Characterization of Some New Axially Ligated Indium(III) Macrocyclic Complexes and Their Biological Activities.Bioinorg Chem Appl. 2014;2014:865407. doi: 10.1155/2014/865407. Epub 2014 Jul 21. Bioinorg Chem Appl. 2014. PMID: 25140121 Free PMC article.
-
Synthesis, characterization and spectral properties of substituted tetraphenylporphyrin iron chloride complexes.Molecules. 2011 Apr 6;16(4):2960-70. doi: 10.3390/molecules16042960. Molecules. 2011. PMID: 21471935 Free PMC article.
-
"Axial-Bonding"-Type Hybrid Porphyrin Arrays: Synthesis, Spectroscopy, Electrochemistry, and Singlet State Properties.Inorg Chem. 1999 Nov 1;38(22):4971-4980. doi: 10.1021/ic990326c. Inorg Chem. 1999. PMID: 11671239
-
Spectroscopic studies and biological evaluation of some transition metal complexes of azo Schiff-base ligand derived from (1-phenyl-2,3-dimethyl-4-aminopyrazol-5-one) and 5-((4-chlorophenyl)diazenyl)-2-hydroxybenzaldehyde.Spectrochim Acta A Mol Biomol Spectrosc. 2012 Oct;96:493-500. doi: 10.1016/j.saa.2012.05.053. Epub 2012 Jun 2. Spectrochim Acta A Mol Biomol Spectrosc. 2012. PMID: 22728967
-
Synthesis, spectroscopic studies and inhibitory activity against bacteria and fungi of acyclic and macrocyclic transition metal complexes containing a triamine coumarine Schiff base ligand.Spectrochim Acta A Mol Biomol Spectrosc. 2015 Apr 15;141:223-32. doi: 10.1016/j.saa.2015.01.063. Epub 2015 Feb 2. Spectrochim Acta A Mol Biomol Spectrosc. 2015. PMID: 25681806
Cited by
-
Synthesis, Characterization, PXRD Studies, Theoretical Calculation, and Antitumor Potency Studies of a Novel N,O-Multidentate Chelating Ligand and Its Zr(IV), V(IV), Ru(III), and Cd(II) Complexes.Bioinorg Chem Appl. 2022 Jun 28;2022:2006451. doi: 10.1155/2022/2006451. eCollection 2022. Bioinorg Chem Appl. 2022. PMID: 38435083 Free PMC article.
-
Axially Ligated Zirconium(IV) Tetraphenylporphyrin: Synthesis, Characterization, and Biological Activity.Bioinorg Chem Appl. 2014;2014:543014. doi: 10.1155/2014/543014. Epub 2014 Oct 14. Bioinorg Chem Appl. 2014. PMID: 25371659 Free PMC article.
References
-
- Xue Z, Lee PPS, Wang Y, et al. Further insight into aryl nitration of tetraphenylporphyrin. Tetrahedron. 2011;67(33):6030–6035.
-
- Xu Y, Liu Z, Zhang X, et al. A graphene hybrid material covalently functionalized with porphyrin: synthesis and optical limiting property. Advanced Materials. 2009;21(12):1275–1279.
-
- Natale CD, Monti D, Paolesse R. Chemical sensitivity of porphyrin assemblies. Materials Today. 2010;13(7-8):46–52.
-
- Wathier M, Grinstaff MW. Synthesis and properties of supramolecular ionic networks. Journal of the American Chemical Society. 2008;130(30):9648–9649. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

