"Zebrafishing" for novel genes relevant to the glomerular filtration barrier
- PMID: 24106712
- PMCID: PMC3784067
- DOI: 10.1155/2013/658270
"Zebrafishing" for novel genes relevant to the glomerular filtration barrier
Abstract
Data for genes relevant to glomerular filtration barrier function or proteinuria is continually increasing in an era of microarrays, genome-wide association studies, and quantitative trait locus analysis. Researchers are limited by published literature searches to select the most relevant genes to investigate. High-throughput cell cultures and other in vitro systems ultimately need to demonstrate proof in an in vivo model. Generating mammalian models for the genes of interest is costly and time intensive, and yields only a small number of test subjects. These models also have many pitfalls such as possible embryonic mortality and failure to generate phenotypes or generate nonkidney specific phenotypes. Here we describe an in vivo zebrafish model as a simple vertebrate screening system to identify genes relevant to glomerular filtration barrier function. Using our technology, we are able to screen entirely novel genes in 4-6 weeks in hundreds of live test subjects at a fraction of the cost of a mammalian model. Our system produces consistent and reliable evidence for gene relevance in glomerular kidney disease; the results then provide merit for further analysis in mammalian models.
Figures



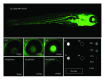

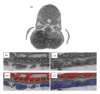

References
-
- Summerton J, Weller D. Morpholino antisense oligomers: design, preparation, and properties. Antisense and Nucleic Acid Drug Development. 1997;7(3):187–195. - PubMed
-
- Hudziak RM, Barofsky E, Barofsky DF, Weller DL, Huang S-B, Weller DD. Resistance of morpholino phosphorodiamidate oligomers to enzymatic degradation. Antisense and Nucleic Acid Drug Development. 1996;6(4):267–272. - PubMed
-
- Yan YL, Miller CT, Nissen RM, et al. A zebrafish sox9 gene required for cartilage morphogenesis. Development. 2002;129(21):5065–5079. - PubMed
-
- Buchardt O, Egholm M, Berg RH, Nielsen PE. Peptide nucleic acids and their potential applications in biotechnology. Trends in Biotechnology. 1993;11(9):384–386. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

