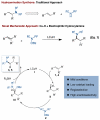
Enantio- and regioselective CuH-catalyzed hydroamination of alkenes
- PMID: 24106781
- PMCID: PMC3874865
- DOI: 10.1021/ja4092819
Enantio- and regioselective CuH-catalyzed hydroamination of alkenes
Abstract
A highly enantio- and regioselective copper-catalyzed hydroamination reaction of alkenes has been developed using diethoxymethylsilane and esters of hydroxylamines. The process tolerates a wide variety of substituted styrenes, including trans-, cis-, and β,β-disubstituted styrenes, to yield α-branched amines. In addition, aliphatic alkenes coupled to generate exclusively the anti-Markovnikov hydroamination products.
Figures
References
-
- Severin R, Doye S. Chem. Soc. Rev. 2007;36:1407. - PubMed
-
- Miki Y, Hirano K, Satoh T, Miura M. Angew. Chem. Int. Ed. 2013;52 doi: 10.1002/anie.201304365. - PubMed
- Pawlas J, Nakao Y, Kawatsura M, Hartwig JF. J. Am. Chem. Soc. 2002;124:3669. - PubMed
- Kawatsura M, Hartwig JF. Organometallics. 2001;20:1960.
- Senn HM, Blochl PE, Togni A. J. Am. Chem. Soc. 2000;122:4098.
- Muller TE, Beller M. Chem. Rev. 1998;98:675. - PubMed
- Muller TE, Hultzch KC, Yus M, Foubelo F, Tada M. Chem. Rev. 2006;108:3795. - PubMed
- Dorta R;, Egli P, Zurcher F, Togni A. J. Am. Chem. Soc. 1997;119:10857.
- Casalnuovo AL, Calabrese JC, Milstein D. J. Am. Chem. Soc. 1988;110:6738.
- Zhou J, Hartwig JF. J. Am. Chem. Soc. 2008;130:12220. - PubMed
- Lober O, Kawatsura M, Hartwig JF. J. Am. Chem. Soc. 2001;123:4366. - PubMed
- Li K, Horton PN, Hursthouse MB, Hii KK. J. Organomet. Chem. 2003;665:250.
- Hu A, Ogasawara M, Sakamoto T, Okada A;, Nakajima K, Takahashi T, Lin W. Adv. Synth. Catal. 2006;348:2051.
- Nettekoven U, Hartwig JF. J. Am. Chem. Soc. 2002;124:1166. - PubMed
-
-
Utsunomiya M, Kuwano R, Kawatsura M, Hartwig JF. J. Am. Chem. Soc. 2003;125:5608. Kawatsura M, Hartwig JF. J. Am. Chem. Soc. 2000;122:9546. (c) The work of Miura (see ref. a), which was reported concurrent to the preparation to this manuscript, was successful in the asymmetric hydroamination of β–substituted styrenes.
-
-
-
Nobis M, Drieβen.Hölscher B. Angew. Chem. Int. Ed. 2001;40:3983. (b) Lalic has described an elegant Cu-catalyzed hydroboration/transmetallation sequence to primary amines: Rucker RP, Whittaker AM, Dang H, Lalic G. J. Am. Chem. Soc. 2012;134:6571.
-
-
-
Appella DH, Moritani Y, Shintani R, Ferreira EM, Buchwald SL. J. Am. Chem. Soc. 1999;121:9473. Moritani Y, Appella DH, Jurkauskas V, Buchwald SL. J. Am. Chem. Soc. 2000;122:6797. Yun J, Buchwald SL. Org. Lett. 2001;3:1129. Jurkauskas V, Buchwald SL. J. Am. Chem. Soc. 2002;124:2892. Rainka MP, Aye Y, Buchwald SL. Proc. Natl. Ac. Sci. U.S.A. 2004;101:5821. For other examples of Cu.catalyzed asymmetric hydrogenation reactions of vinyl heteroarenes (See ref. f-g). Rupnicki L, Saxena A, Lam HW. J. Am. Chem. Soc. 2009;131:10386. Saxena A, Choi B, Lam HW. J. Am. Chem. Soc. 2012;134:8428.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources