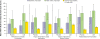Self-Reported quality of life in adults with attention-deficit/hyperactivity disorder and executive function impairment treated with lisdexamfetamine dimesylate: a randomized, double-blind, multicenter, placebo-controlled, parallel-group study
- PMID: 24106804
- PMCID: PMC3854089
- DOI: 10.1186/1471-244X-13-253
Self-Reported quality of life in adults with attention-deficit/hyperactivity disorder and executive function impairment treated with lisdexamfetamine dimesylate: a randomized, double-blind, multicenter, placebo-controlled, parallel-group study
Abstract
Background: This study examined the effects of lisdexamfetamine dimesylate (LDX) on quality of life (QOL) in adults with attention-deficit/hyperactivity disorder (ADHD) and clinically significant executive function deficits (EFD).
Methods: This report highlights QOL findings from a 10-week randomized placebo-controlled trial of LDX (30-70 mg/d) in adults (18-55 years) with ADHD and EFD (Behavior Rating Inventory of EF-Adult, Global Executive Composite [BRIEF-A GEC] ≥65). The primary efficacy measure was the self-reported BRIEF-A; a key secondary measure was self-reported QOL on the Adult ADHD Impact Module (AIM-A). The clinician-completed ADHD Rating Scale version IV (ADHD-RS-IV) with adult prompts and Clinical Global Impressions-Severity (CGI-S) were also employed. The Adult ADHD QoL (AAQoL) was added while the study was in progress. A post hoc analysis examined the subgroup having evaluable results from both AIM-A and AAQoL.
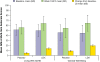
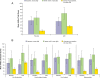
Results: Of 161 randomized (placebo, 81; LDX, 80), 159 were included in the safety population. LDX improved AIM-A multi-item domain scores versus placebo; LS mean difference for Performance and Daily Functioning was 21.6 (ES, 0.93, P<.0001); Impact of Symptoms: Daily Interference was 14.9 (ES, 0.62, P<.0001); Impact of Symptoms: Bother/Concern was 13.5 (ES, 0.57, P=.0003); Relationships/Communication was 7.8 (ES, 0.31, P=.0302); Living With ADHD was 9.1 (ES, 0.79, P<.0001); and General Well-Being was 10.8 (ES, 0.70, P<.0001). AAQoL LS mean difference for total score was 21.0; for subscale: Life Productivity was 21.0; Psychological Health was 12.1; Life Outlook was 12.5; and Relationships was 7.3. In a post hoc analysis of participants with both AIM-A and AAQoL scores, AIM-A multi-item subgroup analysis scores numerically improved with LDX, with smaller difference for Impact of Symptoms: Daily Interference. The safety profile of LDX was consistent with amphetamine use in previous studies.
Conclusions: Overall, adults with ADHD/EFD exhibited self-reported improvement on QOL, using the AIM-A and AAQoL scales in line with medium/large ES; these improvements were paralleled by improvements in EF and ADHD symptoms. The safety profile of LDX was similar to previous studies.
Trial registration: ClinicalTrials.gov, NCT01101022.
Figures
References
-
- Kessler RC, Adler L, Barkley R, Biederman J, Conners CK, Demler O, Faraone SV, Greenhill LL, Howes MJ, Secnik K. et al.The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163(4):716–723. doi: 10.1176/appi.ajp.163.4.716. - DOI - PMC - PubMed
-
- Brown TE. Executive functions and attention deficit hyperactivity disorder: implications of two conflicting views. Int J Disability Develop Educ. 2006;53(1):35–46. doi: 10.1080/10349120500510024. - DOI
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical