Simple catalytic mechanism for the direct coupling of α-carbonyls with functionalized amines: a one-step synthesis of Plavix
- PMID: 24107144
- PMCID: PMC3934301
- DOI: 10.1021/ja4096472
Simple catalytic mechanism for the direct coupling of α-carbonyls with functionalized amines: a one-step synthesis of Plavix
Abstract
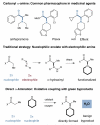
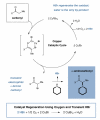
The direct α-amination of ketones, esters, and aldehydes has been accomplished via copper catalysis. In the presence of catalytic copper(II) bromide, a diverse range of carbonyl and amine substrates undergo fragment coupling to produce synthetically useful α-amino-substituted motifs. The transformation is proposed to proceed via a catalytically generated α-bromo carbonyl species; nucleophilic displacement of the bromide by the amine then delivers the α-amino carbonyl adduct while the catalyst is reconstituted. The practical value of this transformation is highlighted through one-step syntheses of two high-profile pharmaceutical agents, Plavix and amfepramone.
Figures
References
-
- Meltzer PC, Butler D, Deschamps JR, Madras BK. J. Med. Chem. 2006;49:1420–1432. - PMC - PubMed
- Carrol FI, Blough BE, Abraham P, Mills AC, Holleman JA, Wolchenhauer SA, Decker AM, Landavazo AK, McElroy T, Navarro HA, Gatch MB, Forster MJ. J. Med. Chem. 2009;52:6768–6781. - PubMed
- Bouteiller C, Becerril-Ortega J, Marchand P, Nicole O, Barre L, Buisson A, Perrio C. Org. Biomol. Chem. 2010;8:1111–1120. - PubMed
- Meyers MC, Wang J-L, Iera JA, Bang J-K, Hara T, Saito S, Zambetti GP, Appella DH. J. Am. Chem. Soc. 2005;127:6152–6153. - PubMed
- Ando R, Sakaki T, Morinaka Y, Takahashi C, Tamao Y. 1994. EP 603769 A1 19940629. - PubMed
-
-
For a review of such methods, see: Janey JM. Angew. Chem. Int. Ed. 2005;44:4292–3300. Vilaivan T, Bhanthumnavin W. Molecules. 2010;15:917–958.
-
-
- Matsuda N, Hirano K, Satoh T, Miura M. Angew. Chem. Int. Ed. 2012;51:11827–11831. - PubMed
- Miura T, Morimoto M, Murakami M. Org. Lett. 2012;14:5214–5217. - PubMed
- Wei Y, Lin S, Liang F. Org. Lett. 2012;14:4202–4205. - PubMed
- Lamani M, Prabhu KR. Chem. Eur. J. 2012;18:14638–14642. - PubMed
- Tian J-S, Loh T-P. Chem. Commun. 2011;47:5458–5460. - PubMed
- Tian J-S, Ng KWJ, Wong J-R, Loh T-P. Angew. Chem. Int. Ed. 2012;51:9105–9109. - PubMed
-
- Guram AS, Rennels RA, Buchwald SL. Angew. Chem. Int. Ed. 1995;34:1348–1350.
- Louie J, Hartwig JF. Tetrahedron Lett. 1995;36:3609–3612.
-
- Chan DMT, Monaco KL, Wang RP, Winters MP. Tetrahedron Lett. 1998;39:2933.
- Lam P, Clark CG, Saubern S, Adams J, Winters MP, Chan DM, Combs T, A. Tetrahedron Lett. 1998;39:2941.
- Lam P, Vincent G, Bonne D, Clark CG. Tetrahedron Lett. 2003;44:4927.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources