Glucocorticoid-induced TNF receptor family-related protein ligand regulates the migration of monocytes to the inflamed intestine
- PMID: 24107315
- PMCID: PMC3868826
- DOI: 10.1096/fj.13-236505
Glucocorticoid-induced TNF receptor family-related protein ligand regulates the migration of monocytes to the inflamed intestine
Abstract
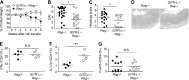
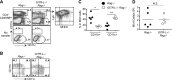
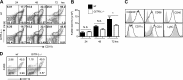
Glucocorticoid-induced TNF receptor family-related protein (GITR) regulates the function of both T cells and antigen-presenting cells (APCs), while the function of GITR ligand (GITR-L) is largely unknown. Here we evaluate the role of GITR-L, whose expression is restricted to APCs, in the development of enterocolitis. On injecting naive CD4(+) T cells, GITR-L(-/-)Rag(-/-) mice develop a markedly milder colitis than Rag(-/-) mice, which correlates with a 50% reduction of Ly6C(+)CD11b(+)MHCII(+) macrophages in the lamina propria and mesenteric lymph nodes. The same result was observed in αCD40-induced acute colitis and during peritonitis, suggesting an altered monocyte migration. In line with these observations, the number of nondifferentiated monocytes was approximately 3-fold higher in the spleen of GITR-L(-/-)Rag(-/-) mice than in Rag(-/-) mice after αCD40 induction. Consistent with the dynamic change in the formation of an active angiotensin II type 1 receptor (AT1) dimer in GITR-L(-/-) splenic monocytes during intestinal inflammation, the migratory capability of splenic monocytes from GITR-L-deficient mice was impaired in an in vitro transwell migration assay. Conversely, αGITR-L reduces the number of splenic Ly6C(hi) monocytes, concomitantly with an increase in AT1 dimers. We conclude that GITR-L regulates the number of proinflammatory macrophages in sites of inflammation by controlling the egress of monocytes from the splenic reservoir.
Keywords: GITR-L; TNFSF18; experimental colitis; macrophage.
Figures
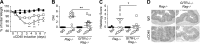




References
-
- Denning T. L., Wang Y. C., Patel S. R., Williams I. R., Pulendran B. (2007) Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat. Immunol. 8, 1086–1094 - PubMed
-
- Varol C., Vallon-Eberhard A., Elinav E., Aychek T., Shapira Y., Luche H., Fehling H. J., Hardt W. D., Shakhar G., Jung S. (2009) Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity 31, 502–512 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

