Nicotine dependence produces hyperalgesia: role of corticotropin-releasing factor-1 receptors (CRF1Rs) in the central amygdala (CeA)
- PMID: 24107576
- PMCID: PMC4144034
- DOI: 10.1016/j.neuropharm.2013.09.025
Nicotine dependence produces hyperalgesia: role of corticotropin-releasing factor-1 receptors (CRF1Rs) in the central amygdala (CeA)
Abstract
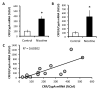
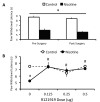
Because tobacco use has a large negative health and financial impact on society, it is critical to identify the factors that drive excessive use. These factors include the aversive withdrawal symptoms that manifest upon cessation of tobacco use, and may include increases in nociceptive processing. Corticotropin-releasing factor (CRF) signalling in the central amygdala (CeA) has been attributed an important role in: (1) central processing of pain, (2) excessive nicotine use that results in nicotine dependence, and (3) in mediating the aversive symptoms that manifest following cessation of tobacco exposure. Here, we describe three experiments in which the main hypothesis was that CRF/CRF1 receptor (CRF1R) signalling in the CeA mediates nicotine withdrawal-induced increases in nociceptive sensitivity in rats that are dependent on nicotine. In Experiment 1, nicotine-dependent rats withdrawn from chronic intermittent (14-h/day) nicotine vapor exhibited decreased hind paw withdrawal latencies in response to a painful thermal stimulus in the Hargreaves test, and this effect was attenuated by systemic administration of the CRF1R antagonist, R121919. In Experiment 2, nicotine-dependent rats withdrawn from nicotine vapor exhibited robust increases in mRNA for CRF and CRF1Rs in CeA. In Experiment 3, intra-CeA administration of R121919 reduced thermal nociception only in nicotine-dependent rats. Collectively, these results suggest that nicotine dependence increases CRF/CRF1R signalling in the CeA that mediates withdrawal-induced increases in sensitivity to a painful stimulus. Future studies will build on these findings by exploring the hypothesis that nicotine withdrawal-induced reduction in pain thresholds drive excessive nicotine use via CRF/CRF1R signalling pathways.
Keywords: CRF1 receptor; Hargreaves; Nicotine dependence; R121919; Withdrawal.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures

References
-
- World Health Organization . WHO | Data and statistics; 2012.
-
- Adhikari B. Smoking-attributable mortality, years of potential life lost, and productivity. MMWR Morb Mortal Wkly Rep. 2008;57(45):1226–1228. - PubMed
-
- Arborelius L, Owens M, Plotsky P, Nemeroff C. The role of corticotropin-releasing factor in depression and anxiety disorders. Journal of Endocrinology. 1999;160:1–10. - PubMed
-
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annual Reviews of Pharmacology and Toxicology. 2004;44:525–557. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

