A critical role for macrophages near axotomized neuronal cell bodies in stimulating nerve regeneration
- PMID: 24107955
- PMCID: PMC3792461
- DOI: 10.1523/JNEUROSCI.3319-12.2013
A critical role for macrophages near axotomized neuronal cell bodies in stimulating nerve regeneration
Abstract
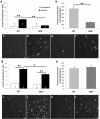
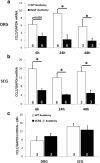
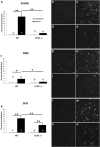
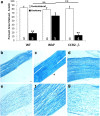
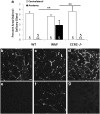
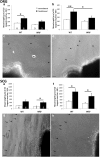
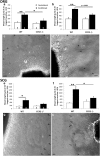
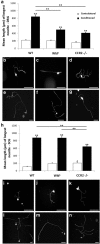
Macrophages have been implicated in peripheral nerve regeneration for some time, supposedly through their involvement in Wallerian degeneration, the process by which the distal nerve degenerates after axotomy and is cleared by phagocytosis. Thus, in several studies in which macrophage accumulation in the distal nerve was reduced and Wallerian degeneration inhibited, regeneration was delayed. However, this interpretation ignores the more recent findings that macrophages also accumulate around axotomized cell bodies. The function of macrophage action at this second site has not been clear. In two mutant strains of mice, the slow Wallerian degeneration (Wld(s)) mouse and the chemokine receptor CCR2 knock-out mouse, we report that macrophage accumulation after axotomy was abolished in both the dorsal root ganglion (DRG) and the distal sciatic nerve. To measure neurite outgrowth, DRG neurons were given a conditioning lesion, and outgrowth was measured in vitro 7 d later in the absence of the distal nerve segment. The increased growth normally seen after a conditioning lesion did not occur or was reduced in Wld(s) or CCR2(-/-) mice. In the superior cervical ganglion (SCG), particularly in Wld(s) mice, macrophage accumulation was reduced but not abolished after axotomy. In SCG neurons from Wld(s) mice, the conditioning lesion response was unchanged; however, in CCR2(-/-) mice in which the effect on macrophage accumulation was greater, SCG neurite outgrowth was significantly reduced. These results indicate that macrophages affect neurite outgrowth by acting at the level of peripheral ganglia in addition to any effects they might produce by facilitation of Wallerian degeneration.
Figures

References
-
- Barrette B, Calvo E, Vallières N, Lacroix S. Transcriptional profiling of the injured sciatic nerve of mice carrying the Wld(S) mutant gene: identification of genes involved in neuroprotection, neuroinflammation, and nerve regeneration. Brain Behav Immun. 2010;24:1254–1267. doi: 10.1016/j.bbi.2010.07.249. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
- R56 DK097223/DK/NIDDK NIH HHS/United States
- P30EY11373/EY/NEI NIH HHS/United States
- NS067431/NS/NINDS NIH HHS/United States
- R25 GM075207/GM/NIGMS NIH HHS/United States
- T32 NS077888/NS/NINDS NIH HHS/United States
- GM075207/GM/NIGMS NIH HHS/United States
- T32 NS067431/NS/NINDS NIH HHS/United States
- NS017512/NS/NINDS NIH HHS/United States
- P30 EY011373/EY/NEI NIH HHS/United States
- R01 NS017512/NS/NINDS NIH HHS/United States
- R01 DK097223/DK/NIDDK NIH HHS/United States
- R01 NS095017/NS/NINDS NIH HHS/United States
- DK097223/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
