Arteriolar niches maintain haematopoietic stem cell quiescence
- PMID: 24107994
- PMCID: PMC3821873
- DOI: 10.1038/nature12612
Arteriolar niches maintain haematopoietic stem cell quiescence
Abstract
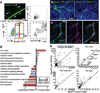
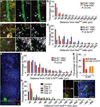
Cell cycle quiescence is a critical feature contributing to haematopoietic stem cell (HSC) maintenance. Although various candidate stromal cells have been identified as potential HSC niches, the spatial localization of quiescent HSCs in the bone marrow remains unclear. Here, using a novel approach that combines whole-mount confocal immunofluorescence imaging techniques and computational modelling to analyse significant three-dimensional associations in the mouse bone marrow among vascular structures, stromal cells and HSCs, we show that quiescent HSCs associate specifically with small arterioles that are preferentially found in endosteal bone marrow. These arterioles are ensheathed exclusively by rare NG2 (also known as CSPG4)(+) pericytes, distinct from sinusoid-associated leptin receptor (LEPR)(+) cells. Pharmacological or genetic activation of the HSC cell cycle alters the distribution of HSCs from NG2(+) periarteriolar niches to LEPR(+) perisinusoidal niches. Conditional depletion of NG2(+) cells induces HSC cycling and reduces functional long-term repopulating HSCs in the bone marrow. These results thus indicate that arteriolar niches are indispensable for maintaining HSC quiescence.
Figures



Comment in
-
A new image of the hematopoietic stem cell vascular niche.Cell Stem Cell. 2013 Nov 7;13(5):514-6. doi: 10.1016/j.stem.2013.10.012. Cell Stem Cell. 2013. PMID: 24209758 Free PMC article.
References
References (ON LINE ONLY)
-
- Kawamoto T. Use of a new adhesive film for the preparation of multi-purpose fresh-frozen sections from hard tissues, whole-animals, insects and plants. Arch Histol Cytol. 2003;66:123–143. - PubMed
-
- Chiang EY, Hidalgo A, Chang J, Frenette PS. Imaging receptor microdomains on leukocyte subsets in live mice. Nat Methods. 2007;4:219–222. - PubMed
-
- Miller CL, Dykstra B, Eaves CJ. Characterization of mouse hematopoietic stem and progenitor cells. Curr Protoc Immunol. 2008;Chapter 22(Unit 22B):22. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

