Connexin defects underlie arrhythmogenic right ventricular cardiomyopathy in a novel mouse model
- PMID: 24108106
- PMCID: PMC3919010
- DOI: 10.1093/hmg/ddt508
Connexin defects underlie arrhythmogenic right ventricular cardiomyopathy in a novel mouse model
Abstract
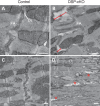
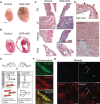
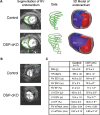
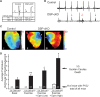
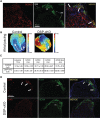
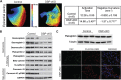
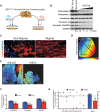
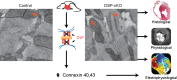
Arrhythmogenic right ventricular cardiomyopathy (ARVC) termed a 'disease of the desmosome' is an inherited cardiomyopathy that recently underwent reclassification owing to the identification of left-dominant and biventricular disease forms. Homozygous loss-of-function mutations in the desmosomal component, desmoplakin, are found in patients exhibiting a biventricular form of ARVC; however, no models recapitulate the postnatal hallmarks of the disease as seen in these patients. To gain insights into the homozygous loss-of-function effects of desmoplakin in the heart, we generated cardiomyocyte-specific desmoplakin-deficient mice (DSP-cKO) using ventricular myosin light chain-2-Cre mice. Homozygous DSP-cKO mice are viable but display early ultrastructural defects in desmosomal integrity leading to a cardiomyopathy reminiscent of a biventricular form of ARVC, which includes cell death and fibro-fatty replacement within the ventricle leading to biventricular dysfunction, failure and premature death. DSP-cKO mice also exhibited ventricular arrhythmias that are exacerbated with exercise and catecholamine stimulation. Furthermore, DSP-cKO hearts exhibited right ventricular conduction defects associated with loss of connexin 40 expression and electrical wavefront propagation defects associated with loss of connexin 43 expression. Dose-dependent assessment of the effects of loss of desmoplakin in neonatal ventricular cardiomyocytes revealed primary loss of connexin 43 levels, phosphorylation and function independent of the molecular dissociation of the mechanical junction complex and fibro-fatty manifestation associated with ARVC, suggesting a role for desmoplakin as a primary stabilizer of connexin integrity. In summary, we provide evidence for a novel mouse model, which is reminiscent of the postnatal onset of ARVC while highlighting mechanisms underlying a biventricular form of human ARVC.
Figures

References
-
- Sen-Chowdhry S., Morgan R.D., Chambers J.C., McKenna W.J. Arrhythmogenic cardiomyopathy: etiology, diagnosis, and treatment. Annu. Rev. Med. 2010;61:233–253. - PubMed
-
- McKoy G., Protonotarios N., Crosby A., Tsatsopoulou A., Anastasakis A., Coonar A., Norman M., Baboonian C., Jeffery S., McKenna W.J. Identification of a deletion in plakoglobin in arrhythmogenic right ventricular cardiomyopathy with palmoplantar keratoderma and woolly hair (Naxos disease) Lancet. 2000;355:2119–2124. - PubMed
-
- Rampazzo A., Nava A., Malacrida S., Beffagna G., Bauce B., Rossi V., Zimbello R., Simionati B., Basso C., Thiene G., et al. Mutation in human desmoplakin domain binding to plakoglobin causes a dominant form of arrhythmogenic right ventricular cardiomyopathy. Am. J. Hum. Genet. 2002;71:1200–1206. - PMC - PubMed
-
- Gerull B., Heuser A., Wichter T., Paul M., Basson C.T., McDermott D.A., Lerman B.B., Markowitz S.M., Ellinor P.T., MacRae C.A., et al. Mutations in the desmosomal protein plakophilin-2 are common in arrhythmogenic right ventricular cardiomyopathy. Nat. Genet. 2004;36:1162–1164. - PubMed
-
- Pilichou K., Nava A., Basso C., Beffagna G., Bauce B., Lorenzon A., Frigo G., Vettori A., Valente M., Towbin J., et al. Mutations in desmoglein-2 gene are associated with arrhythmogenic right ventricular cardiomyopathy. Circulation. 2006;113:1171–1179. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

