Functional outcomes in pediatric severe sepsis: further analysis of the researching severe sepsis and organ dysfunction in children: a global perspective trial
- PMID: 24108117
- PMCID: PMC4080839
- DOI: 10.1097/PCC.0b013e3182a551c8
Functional outcomes in pediatric severe sepsis: further analysis of the researching severe sepsis and organ dysfunction in children: a global perspective trial
Abstract
Objectives: To evaluate risk factors for poor functional outcome in 28-day survivors after an episode of severe sepsis.
Design: Retrospective cohort study examining data from the Researching Severe Sepsis and Organ Dysfunction in Children: A Global Perspective trial (NCT00049764).
Setting: One hundred and four pediatric centers in 18 countries.
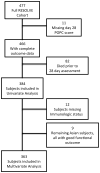
Subjects: Children with severe sepsis who required both vasoactive-inotropic infusions and mechanical ventilation and who survived to 28 days (n = 384).
Interventions: None.
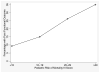
Measurements and main results: Poor functional outcome was defined as a Pediatric Overall Performance Category score greater than or equal to 3 and an increase from baseline when measured 28 days after trial enrollment. Median Pediatric Overall Performance Category at enrollment was 1 (interquartile range, 1-2). Median Pediatric Overall Performance Category at 28 days was 2 (interquartile range, 1-4). Thirty-four percent of survivors had decline in their functional status at 28 days, and 18% were determined to have a "poor" functional outcome. Hispanic ethnicity was associated with poor functional outcome compared to the white referent group (risk ratio = 1.9; 95% CI: 1.0-3.0). Clinical factors associated with increased risk of poor outcome included CNS and intra-abdominal infection sources compared to the lung infection referent category (risk ratio = 3.3; 95% CI: 1.4-5.6 and 2.4; 95% CI: 1.0-4.5, respectively); a history of recent trauma (risk ratio = 3.9; 95% CI: 1.4-5.4); receipt of cardiopulmonary resuscitation prior to enrollment (risk ratio = 5.1; 95% CI: 2.9-5.7); and baseline Pediatric Risk of Mortality III score of 20-29 (risk ratio = 2.8; 95% CI: 1.2-5.2) and Pediatric Risk of Mortality III greater than or equal to 30 (risk ratio = 4.5; 95% CI: 1.6-8.0) compared to the referent group with Pediatric Risk of Mortality III scores of 0-9.
Conclusions: In this sample of 28-day survivors of pediatric severe sepsis diminished functional status was common. This analysis provides evidence that particular patient characteristics and aspects of an individual's clinical course are associated with poor functional outcome 28 days after onset of severe sepsis. These characteristics may provide opportunity for intervention in order to improve functional outcome in pediatric patients with severe sepsis. Decline in functional status 28 days after onset of severe sepsis is a frequent and potentially clinically meaningful event. Utilization of functional status as the primary outcome in future pediatric sepsis clinical trials should be considered.
Figures

Comment in
-
Functional outcomes for children with severe sepsis: is a "good save" good enough?Pediatr Crit Care Med. 2013 Nov;14(9):893-4. doi: 10.1097/PCC.0b013e3182a551e9. Pediatr Crit Care Med. 2013. PMID: 24226554 No abstract available.
References
-
- Goldstein B, Giroir B, Randolph AG Sepsis ICC on P. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6(1):2–8. - PubMed
-
- Watson RS, Carcillo JA, Linde-Zwirble WT, et al. The epidemiology of severe sepsis in children in the United States. American Journal of Respiratory and Critical Care Medicine. 2003;167(5):695–701. - PubMed
-
- Watson RS, Carcillo JA. Scope and epidemiology of pediatric sepsis. Pediatr Crit Care Med. 2005;6(3 Suppl):S3–5. - PubMed
-
- Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatr. 1992;121(1):68–74. - PubMed
-
- Fiser DH, Tilford JM, Roberson PK. Relationship of illness severity and length of stay to functional outcomes in the pediatric intensive care unit: a multi-institutional study. Crit Care Med. 2000;28(4):1173–9. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

