Structural and functional characterization of two alpha-synuclein strains
- PMID: 24108358
- PMCID: PMC3826637
- DOI: 10.1038/ncomms3575
Structural and functional characterization of two alpha-synuclein strains
Abstract
α-Synuclein aggregation is implicated in a variety of diseases including Parkinson's disease, dementia with Lewy bodies, pure autonomic failure and multiple system atrophy. The association of protein aggregates made of a single protein with a variety of clinical phenotypes has been explained for prion diseases by the existence of different strains that propagate through the infection pathway. Here we structurally and functionally characterize two polymorphs of α-synuclein. We present evidence that the two forms indeed fulfil the molecular criteria to be identified as two strains of α-synuclein. Specifically, we show that the two strains have different structures, levels of toxicity, and in vitro and in vivo seeding and propagation properties. Such strain differences may account for differences in disease progression in different individuals/cell types and/or types of synucleinopathies.
Figures
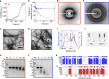
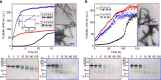
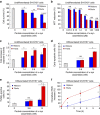
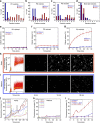
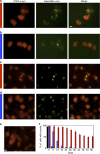
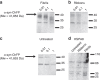
References
-
- Ross C. A. & Poirier M. A. Protein aggregation and neurodegenerative disease. Nat. Med. 10 Suppl, S10-7 (2004). - PubMed
-
- Goedert M. Alpha-synuclein and neurodegenerative diseases. Nat. Rev. Neurosci. 2, 492–501 (2001). - PubMed
-
- Lee H. J., Choi C. & Lee S. J. Membrane-bound alpha-synuclein has a high aggregation propensity and the ability to seed the aggregation of the cytosolic form. J. Biol. Chem. 277, 671–678 (2002). - PubMed
-
- Jensen P. H., Nielsen M. S., Jakes R., Dotti C. G. & Goedert M. Binding of alpha-synuclein to brain vesicles is abolished by familial Parkinson’s disease mutation. J. Biol. Chem. 273, 26292–26294 (1998). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

