Automated determination of metastases in unstructured radiology reports for eligibility screening in oncology clinical trials
- PMID: 24108448
- PMCID: PMC4358809
- DOI: 10.1177/1535370213508172
Automated determination of metastases in unstructured radiology reports for eligibility screening in oncology clinical trials
Abstract
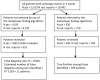
Enrolling adequate numbers of patients that meet protocol eligibility criteria in a timely manner is critical, yet clinical trial accrual continues to be problematic. One approach to meet these accrual challenges is to utilize technology to automatically screen patients for clinical trial eligibility. This manuscript reports on the evaluation of different automated approaches to determine the metastatic status from unstructured radiology reports using the Clinical Trials Eligibility Database Integrated System (CTED). The study sample included all patients (N = 5,523) with radiologic diagnostic studies (N = 10,492) completed in a two-week period. Eight search algorithms (queries) within CTED were developed and applied to radiology reports. The performance of each algorithm was compared to a reference standard which consisted of a physician's review of the radiology reports. Sensitivity, specificity, positive, and negative predicted values were calculated for each algorithm. The number of patients identified by each algorithm varied from 187 to 330 and the number of true positive cases confirmed by physician review ranged from 171 to 199 across the algorithms. The best performing algorithm had sensitivity 94%, specificity 100%, positive predictive value 90%, negative predictive value 100%, and accuracy of 99%. Our evaluation process identified the optimal method for rapid identification of patients with metastatic disease through automated screening of unstructured radiology reports. The methods developed using the CTED system could be readily implemented at other institutions to enhance the efficiency of research staff in the clinical trials eligibility screening process.
Keywords: Clinical trials; automation; eligibility screening; information extraction; metastases; radiology reports.
Figures
References
-
- Schroen AT, Petroni GR, Wang H, Gray R, Wang XF, Cronin W, Sargent DJ, Benedetti J, Wickerham DL, Djulbegovic B, Slingluff CL., Jr. Preliminary evaluation of factors associated with premature trial closure and feasibility of accrual benchmarks in phase III oncology trials. Clin.Trials. 2010;7:312–21. - PMC - PubMed
-
- Nass SJ, Moses HL, Mendelsohn J. A National Cancer Clinical Trials System for the 21st Century: Reinvigorating the NCI Cooperative Group Program. The National Accademies Press; Washington DC: 2010. - PubMed
-
- Wang-Gillam A, Williams K, Novello S, Gao F, Scagliotti GV, Govindan R. Time to activate lung cancer clinical trials and patient enrollment: a representative comparison study between two academic centers across the atlantic. J.Clin.Oncol. 2010;28:3803–07. - PubMed
-
- Schroen AT, Petroni GR, Wang H, Thielen MJ, Gray R, Benedetti J, Wang X, Sargent DJ, Wickerham DL, Cronin WM, Djulbegovic B, Slingluff CL. Achieving Sufficient Accrual to Address the Primary Endpoint in Phase III Clinical Trials from US Cooperative Oncology Groups. Clin.Cancer Res. 2011;18:256–62. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical