Small-animal whole-body photoacoustic tomography: a review
- PMID: 24108456
- PMCID: PMC3965654
- DOI: 10.1109/TBME.2013.2283507
Small-animal whole-body photoacoustic tomography: a review
Abstract
With the wide use of small animals for biomedical studies, in vivo small-animal whole-body imaging plays an increasingly important role. Photoacoustic tomography (PAT) is an emerging whole-body imaging modality that shows great potential for preclinical research. As a hybrid technique, PAT is based on the acoustic detection of optical absorption from either endogenous tissue chromophores, such as oxyhemoglobin and deoxyhemoglobin, or exogenous contrast agents. Because ultrasound scatters much less than light in tissue, PAT generates high-resolution images in both the optical ballistic and diffusive regimes. Using near-infrared light, which has relatively low blood absorption, PAT can image through the whole body of small animals with acoustically defined spatial resolution. Anatomical and vascular structures are imaged with endogenous hemoglobin contrast, while functional and molecular images are enabled by the wide choice of exogenous optical contrasts. This paper reviews the rapidly growing field of small-animal whole-body PAT and highlights studies done in the past decade.
Figures



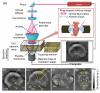


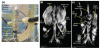


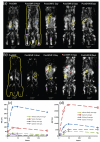

References
-
- Kiessling F, Pichler BJ. Small Animal Imaging: Basics and Practical Guide. Springer Verlag; 2010.
-
- Baker M. Whole-animal imaging: The whole picture. Nature. 2010;463:977–980. - PubMed
-
- Benveniste H, Blackband S. MR microscopy and high resolution small animal MRI: applications in neuroscience research. Progress in Neurobiology. 2002;67:393–420. - PubMed
-
- Brenner DJ, Hall EJ. Computed Tomography - An Increasing Source of Radiation Exposure. New England Journal of Medicine. 2007;357:2277–2284. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 CA134539/CA/NCI NIH HHS/United States
- R01CA134539/CA/NCI NIH HHS/United States
- R01CA157277/CA/NCI NIH HHS/United States
- U54 CA136398/CA/NCI NIH HHS/United States
- U54CA136398/CA/NCI NIH HHS/United States
- R01CA159959/CA/NCI NIH HHS/United States
- DP1 EB016986/EB/NIBIB NIH HHS/United States
- R01 EB016963/EB/NIBIB NIH HHS/United States
- R01 CA157277/CA/NCI NIH HHS/United States
- R01 CA159959/CA/NCI NIH HHS/United States
- R01 EB010049/EB/NIBIB NIH HHS/United States
- R01 EB008085/EB/NIBIB NIH HHS/United States
- DP1 EB016986/DP/NCCDPHP CDC HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

