Exploring alternative biomaterials for diagnosis of pulmonary tuberculosis in HIV-negative patients by use of the GeneXpert MTB/RIF assay
- PMID: 24108610
- PMCID: PMC3838083
- DOI: 10.1128/JCM.01743-13
Exploring alternative biomaterials for diagnosis of pulmonary tuberculosis in HIV-negative patients by use of the GeneXpert MTB/RIF assay
Abstract
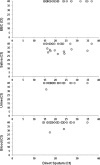
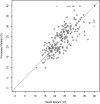
The utility of the GeneXpert MTB/RIF (Xpert) assay for detection of Mycobacterium tuberculosis in sputum samples has been extensively studied. However, the performance of the Xpert assay as applied to other readily accessible body fluids such as exhaled breath condensate (EBC), saliva, urine, and blood has not been established. We used the Xpert assay to test EBC, saliva, urine, and blood samples from HIV-negative, smear- and culture-positive pulmonary tuberculosis (TB) patients for the presence of M. tuberculosis. To compare the ability of the assay to perform bacterial load measurements on sputum samples with versus without sample processing, the assay was also performed on paired direct and processed sputum samples from each patient. The Xpert assay detected M. tuberculosis in none of the 26 EBC samples (sensitivity, 0.0%; 95% confidence interval [95% CI], 0.0%, 12.9%), 10 of the 26 saliva samples (sensitivity, 38.5%; 95% CI, 22.4%, 57.5%), 1 of 26 urine samples (sensitivity, 3.8%; 95% CI, 0.7%, 18.9%), and 2 of 24 blood samples (sensitivity, 8.3%; 95% CI, 2.3%, 25.8%). For bacterial load measurements in the different types of sputum samples, the cycle thresholds of the two M. tuberculosis-positive sputum types were well correlated (Spearman correlation of 0.834). This study demonstrates that the Xpert assay should not be routinely used to detect M. tuberculosis in EBC, saliva, urine, or blood samples from HIV-negative patients suspected of having pulmonary tuberculosis. As a test of bacterial load, the assay produced similar results when used to test direct versus processed sputum samples. Sputum remains the optimal sample type for diagnosing pulmonary tuberculosis in HIV-negative patients with the Xpert assay.
Figures
References
-
- Horvath I, Hunt J, Barnes PJ, Alving K, Antczak A, Baraldi E, Becher G, van Beurden WJ, Corradi M, Dekhuijzen R, Dweik RA, Dwyer T, Effros R, Erzurum S, Gaston B, Gessner C, Greening A, Ho LP, Hohlfeld J, Jobsis Q, Laskowski D, Loukides S, Marlin D, Montuschi P, Olin AC, Redington AE, Reinhold P, van Rensen EL, Rubinstein I, Silkoff P, Toren K, Vass G, Vogelberg C, Wirtz H, ATS/ERS Task Force on Exhaled Breath Condensate 2005. Exhaled breath condensate: methodological recommendations and unresolved questions. Eur. Respir. J. 26:523–548 - PubMed
-
- Montuschi P. 2007. Analysis of exhaled breath condensate in respiratory medicine: methodological aspects and potential clinical applications. Ther. Adv. Respir. Dis. 1:5–23 - PubMed
-
- Schreiber J, Meyer C, Rusch-Gerdes S, Richter E, Beck H, Fischer JF, Rosahl W. 2002. Mycobacterium tuberculosis gene-amplification in breath condensate of patients with lung tuberculosis. Eur. J. Med. Res. 7:290–291 - PubMed
-
- Chiappin S, Antonelli G, Gatti R, De Palo EF. 2007. Saliva specimen: a new laboratory tool for diagnostic and basic investigation. Clin. Chim. Acta 383:30–40 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

