Fatal measles virus infection prevented by brain-penetrant fusion inhibitors
- PMID: 24109233
- PMCID: PMC3838268
- DOI: 10.1128/JVI.02436-13
Fatal measles virus infection prevented by brain-penetrant fusion inhibitors
Abstract
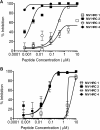
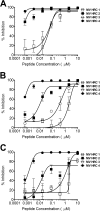
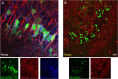
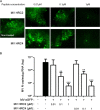
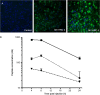
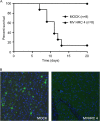
Measles virus (MV) infection causes an acute childhood disease that can include infection of the central nervous system and can rarely progress to severe neurological disease for which there is no specific treatment. We generated potent antiviral peptide inhibitors of MV entry and spreading and MV-induced cell fusion. Dimers of MV-specific peptides derived from the C-terminal heptad repeat region of the MV fusion protein, conjugated to cholesterol, efficiently protect SLAM transgenic mice from fatal MV infection. Fusion inhibitors hold promise for the prophylaxis of MV infection in unvaccinated and immunocompromised people, as well as potential for the treatment of grave neurological complications of measles.
Figures

References
-
- Simons E, Ferrari M, Fricks J, Wannemuehler K, Anand A, Burton A, Strebel P. 2012. Assessment of the 2010 global measles mortality reduction goal: results from a model of surveillance data. Lancet 379:2173–2178 - PubMed
-
- Moss WJ, Griffin DE. 2012. Measles. Lancet 379:153–164 - PubMed
-
- De Serres G, Markowski F, Toth E, Landry M, Auger D, Mercier M, Belanger P, Turmel B, Arruda H, Boulianne N, Ward BJ, Skowronski DM. 2013. The largest measles epidemic in North America in a decade—Quebec, Canada, 2011: contribution of susceptibility, serendipity and super-spreading events on elimination. J. Infect. Dis. 207:990–998 - PubMed
-
- Hosoya M. 2006. Measles encephalitis: direct viral invasion or autoimmune-mediated inflammation? Intern. Med. 45:841–842 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

