Tumor regression grading of gastrointestinal carcinomas after neoadjuvant treatment
- PMID: 24109590
- PMCID: PMC3791673
- DOI: 10.3389/fonc.2013.00262
Tumor regression grading of gastrointestinal carcinomas after neoadjuvant treatment
Abstract
Multimodal therapy concepts have been successfully implemented in the treatment of locally advanced gastrointestinal malignancies. The effects of neoadjuvant chemo- or radiochemotherapy such as scarry fibrosis or resorptive changes and inflammation can be determined by histopathological investigation of the subsequent resection specimen. Tumor regression grading (TRG) systems which aim to categorize the amount of regressive changes after cytotoxic treatment mostly refer onto the amount of therapy induced fibrosis in relation to residual tumor or the estimated percentage of residual tumor in relation to the previous tumor site. Commonly used TRGs for upper gastrointestinal carcinomas are the Mandard grading and the Becker grading system, e.g., and for rectal cancer the Dworak or the Rödel grading system, or other systems which follow similar definitions. Namely for gastro-esophageal carcinomas these TRGs provide important prognostic information since complete or subtotal tumor regression has shown to be associated with better patient's outcome. The prognostic value of TRG may even exceed those of currently used staging systems (e.g., TNM staging) for tumors treated by neoadjuvant therapy. There have been some limitations described regarding interobserver variability especially in borderline cases, which may be improved by standardization of work up of resection specimen and better training of histopathologic determination of regressive changes. It is highly recommended that TRG should be implemented in every histopathological report of neoadjuvant treated gastrointestinal carcinomas. The aim of this review is to disclose the relevance of histomorphological TRG to accomplish an optimal therapy for patients with gastrointestinal carcinomas.
Keywords: gastrointestinal cancer; histopathology; neoadjuvant therapy; tumor regression grade.
Figures
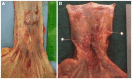
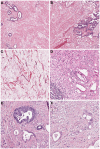

References
-
- Rödel C, Liersch T, Becker H, Fietkau R, Hohenberger W, Hothorn T, et al. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol (2012) 13(7):679–8710.1016/S1470-2045(12)70187-0 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

