Syntaxin 16 is a master recruitment factor for cytokinesis
- PMID: 24109596
- PMCID: PMC3842993
- DOI: 10.1091/mbc.E13-06-0302
Syntaxin 16 is a master recruitment factor for cytokinesis
Abstract
Recently it was shown that both recycling endosome and endosomal sorting complex required for transport (ESCRT) components are required for cytokinesis, in which they are believed to act in a sequential manner to bring about secondary ingression and abscission, respectively. However, it is not clear how either of these complexes is targeted to the midbody and whether their delivery is coordinated. The trafficking of membrane vesicles between different intracellular organelles involves the formation of soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complexes. Although membrane traffic is known to play an important role in cytokinesis, the contribution and identity of intracellular SNAREs to cytokinesis remain unclear. Here we demonstrate that syntaxin 16 is a key regulator of cytokinesis, as it is required for recruitment of both recycling endosome-associated Exocyst and ESCRT machinery during late telophase, and therefore that these two distinct facets of cytokinesis are inextricably linked.
Figures
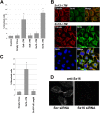

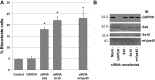

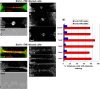



References
-
- Barr FA, Gruneberg U. Cytokinesis: placing and making the final cut. Cell. 2007;131:847–860. - PubMed
-
- Blum R, Stephens DJ, Schulz I. Lumenal targeted GFP, used as a marker of soluble cargo, visualises rapid ERGIC to Golgi traffic by a tubulo-vesicular network. J Cell Sci. 2000;113(Pt 18):3151–3159. - PubMed
-
- Carlton JG, Martin-Serrano J. Parallels between cytokinesis and retroviral budding: a role for the ESCRT machinery. Science. 2007;316:1908–1912. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

