Efficient and rapid derivation of primitive neural stem cells and generation of brain subtype neurons from human pluripotent stem cells
- PMID: 24113065
- PMCID: PMC3808201
- DOI: 10.5966/sctm.2013-0080
Efficient and rapid derivation of primitive neural stem cells and generation of brain subtype neurons from human pluripotent stem cells
Erratum in
- Stem Cells Transl Med. 2013 Dec;2(12):1022
Abstract
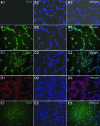
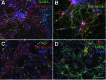
Human pluripotent stem cells (hPSCs), including human embryonic stem cells and human induced pluripotent stem cells, are unique cell sources for disease modeling, drug discovery screens, and cell therapy applications. The first step in producing neural lineages from hPSCs is the generation of neural stem cells (NSCs). Current methods of NSC derivation involve the time-consuming, labor-intensive steps of an embryoid body generation or coculture with stromal cell lines that result in low-efficiency derivation of NSCs. In this study, we report a highly efficient serum-free pluripotent stem cell neural induction medium that can induce hPSCs into primitive NSCs (pNSCs) in 7 days, obviating the need for time-consuming, laborious embryoid body generation or rosette picking. The pNSCs expressed the neural stem cell markers Pax6, Sox1, Sox2, and Nestin; were negative for Oct4; could be expanded for multiple passages; and could be differentiated into neurons, astrocytes, and oligodendrocytes, in addition to the brain region-specific neuronal subtypes GABAergic, dopaminergic, and motor neurons. Global gene expression of the transcripts of pNSCs was comparable to that of rosette-derived and human fetal-derived NSCs. This work demonstrates an efficient method to generate expandable pNSCs, which can be further differentiated into central nervous system neurons and glia with temporal, spatial, and positional cues of brain regional heterogeneity. This method of pNSC derivation sets the stage for the scalable production of clinically relevant neural cells for cell therapy applications in good manufacturing practice conditions.
Keywords: Astrocytes; Cell culture; Nestin; Neural differentiation; Neural induction; Neural stem cell; Neuron; Oligodendrocytes.
Figures





References
-
- Kalyani AJ, Mujtaba T, Rao MS. Expression of EGF receptor and FGF receptor isoforms during neuroepithelial stem cell differentiation. J Neurobiol. 1999;38:207–224. - PubMed
-
- Kalyani A, Hobson K, Rao MS. Neuroepithelial stem cells from the embryonic spinal cord: Isolation, characterization, and clonal analysis. Dev Biol. 1997;186:202–223. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

