Loss of TAK1 increases cell traction force in a ROS-dependent manner to drive epithelial-mesenchymal transition of cancer cells
- PMID: 24113182
- PMCID: PMC3824649
- DOI: 10.1038/cddis.2013.339
Loss of TAK1 increases cell traction force in a ROS-dependent manner to drive epithelial-mesenchymal transition of cancer cells
Abstract
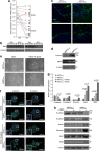
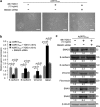
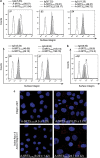
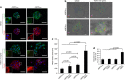
Epithelial-mesenchymal transition (EMT) is a crucial step in tumor progression, and the TGFβ-SMAD signaling pathway as an inductor of EMT in many tumor types is well recognized. However, the role of non-canonical TGFβ-TAK1 signaling in EMT remains unclear. Herein, we show that TAK1 deficiency drives metastatic skin squamous cell carcinoma earlier into EMT that is conditional on the elevated cellular ROS level. The expression of TAK1 is consistently reduced in invasive squamous cell carcinoma biopsies. Tumors derived from TAK1-deficient cells also exhibited pronounced invasive morphology. TAK1-deficient cancer cells adopt a more mesenchymal morphology characterized by higher number of focal adhesions, increase surface expression of integrin α5β1 and active Rac1. Notably, these mutant cells exert an increased cell traction force, an early cellular response during TGFβ1-induced EMT. The mRNA level of ZEB1 and SNAIL, transcription factors associated with mesenchymal phenotype is also upregulated in TAK1-deficient cancer cells compared with control cancer cells. We further show that TAK1 modulates Rac1 and RhoA GTPases activities via a redox-dependent downregulation of RhoA by Rac1, which involves the oxidative modification of low-molecular weight protein tyrosine phosphatase. Importantly, the treatment of TAK1-deficient cancer cells with Y27632, a selective inhibitor of Rho-associated protein kinase and antioxidant N-acetylcysteine augment and hinders EMT, respectively. Our findings suggest that a dysregulated balance in the activation of TGFβ-TAK1 and TGFβ-SMAD pathways is pivotal for TGFβ1-induced EMT. Thus, TAK1 deficiency in metastatic cancer cells increases integrin:Rac-induced ROS, which negatively regulated Rho by LMW-PTP to accelerate EMT.
Figures



References
-
- Mehlen P, Puisieux A. Metastasis: a question of life or death. Nat Rev Cancer. 2006;6:449–458. - PubMed
-
- Ikushima H, Miyazono K. TGFbeta signalling: a complex web in cancer progression. Nat Rev Cancer. 2010;10:415–424. - PubMed
-
- Zavadil J, Böttinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 2005;24:5764–5774. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

