Multisite phosphorylation of c-Jun at threonine 91/93/95 triggers the onset of c-Jun pro-apoptotic activity in cerebellar granule neurons
- PMID: 24113186
- PMCID: PMC3824690
- DOI: 10.1038/cddis.2013.381
Multisite phosphorylation of c-Jun at threonine 91/93/95 triggers the onset of c-Jun pro-apoptotic activity in cerebellar granule neurons
Abstract
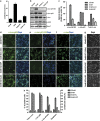
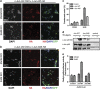
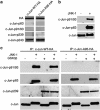
Cerebellar granule cell (CGC) apoptosis by trophic/potassium (TK) deprivation is a model of election to study the interplay of pro-apoptotic and pro-survival signaling pathways in neuronal cell death. In this model, the c-Jun N-terminal kinase (JNK) induces pro-apoptotic genes through the c-Jun/activator protein 1 (AP-1) transcription factor. On the other side, a survival pathway initiated by lithium leads to repression of pro-apoptotic c-Jun/AP-1 target genes without interfering with JNK activity. Yet, the mechanism by which lithium inhibits c-Jun activity remains to be elucidated. Here, we used this model system to study the regulation and function of site-specific c-Jun phosphorylation at the S63 and T91/T93 JNK sites in neuronal cell death. We found that TK-deprivation led to c-Jun multiphosphorylation at all three JNK sites. However, immunofluorescence analysis of c-Jun phosphorylation at single cell level revealed that the S63 site was phosphorylated in all c-Jun-expressing cells, whereas the response of T91/T93 phosphorylation was more sensitive, mirroring the switch-like apoptotic response of CGCs. Conversely, lithium prevented T91T93 phosphorylation and cell death without affecting the S63 site, suggesting that T91T93 phosphorylation triggers c-Jun pro-apoptotic activity. Accordingly, a c-Jun mutant lacking the T95 priming site for T91/93 phosphorylation protected CGCs from apoptosis, whereas it was able to induce neurite outgrowth in PC12 cells. Vice versa, a c-Jun mutant bearing aspartate substitution of T95 overwhelmed lithium-mediate protection of CGCs from TK-deprivation, validating that inhibition of T91/T93/T95 phosphorylation underlies the effect of lithium on cell death. Mass spectrometry analysis confirmed multiphosphorylation of c-Jun at T91/T93/T95 in cells. Moreover, JNK phosphorylated recombinant c-Jun at T91/T93 in a T95-dependent manner. On the basis of our results, we propose that T91/T93/T95 multiphosphorylation of c-Jun functions as a sensitivity amplifier of the JNK cascade, setting the threshold for c-Jun pro-apoptotic activity in neuronal cells.
Figures



Similar articles
-
Negative charged threonine 95 of c-Jun is essential for c-Jun N-terminal kinase-dependent phosphorylation of threonine 91/93 and stress-induced c-Jun biological activity.Int J Biochem Cell Biol. 2008;40(2):307-16. doi: 10.1016/j.biocel.2007.08.001. Epub 2007 Aug 10. Int J Biochem Cell Biol. 2008. PMID: 17920329
-
Identification of JNK-dependent and -independent components of cerebellar granule neuron apoptosis.J Neurochem. 2002 Nov;83(4):992-1001. doi: 10.1046/j.1471-4159.2002.01219.x. J Neurochem. 2002. PMID: 12421372
-
Phosphorylation of c-Jun is necessary for apoptosis induced by survival signal withdrawal in cerebellar granule neurons.J Neurosci. 1998 Jan 15;18(2):751-62. doi: 10.1523/JNEUROSCI.18-02-00751.1998. J Neurosci. 1998. PMID: 9425017 Free PMC article.
-
From JNK to pay dirt: jun kinases, their biochemistry, physiology and clinical importance.IUBMB Life. 2005 Apr-May;57(4-5):283-95. doi: 10.1080/15216540500097111. IUBMB Life. 2005. PMID: 16036612 Review.
-
JNK signaling in apoptosis.Oncogene. 2008 Oct 20;27(48):6245-51. doi: 10.1038/onc.2008.301. Oncogene. 2008. PMID: 18931691 Free PMC article. Review.
Cited by
-
Melatonin signalling in Schwann cells during neuroregeneration.Front Cell Dev Biol. 2022 Oct 10;10:999322. doi: 10.3389/fcell.2022.999322. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36299487 Free PMC article. Review.
-
GPER deletion triggers inhibitory effects in triple negative breast cancer (TNBC) cells through the JNK/c-Jun/p53/Noxa transduction pathway.Cell Death Discov. 2023 Sep 26;9(1):353. doi: 10.1038/s41420-023-01654-0. Cell Death Discov. 2023. PMID: 37749101 Free PMC article.
-
Protein Extraction, Enrichment and MALDI MS and MS/MS Analysis from Bitter Orange Leaves (Citrus aurantium).Molecules. 2020 Mar 25;25(7):1485. doi: 10.3390/molecules25071485. Molecules. 2020. PMID: 32218285 Free PMC article.
-
Influence of Magnesium Degradation on Schwannoma Cell Responses to Nerve Injury Using an In Vitro Injury Model.J Funct Biomater. 2024 Mar 31;15(4):88. doi: 10.3390/jfb15040088. J Funct Biomater. 2024. PMID: 38667545 Free PMC article.
-
Control of neuronal apoptosis by reciprocal regulation of NFATc3 and Trim17.Cell Death Differ. 2015 Feb;22(2):274-86. doi: 10.1038/cdd.2014.141. Epub 2014 Sep 12. Cell Death Differ. 2015. PMID: 25215946 Free PMC article.
References
-
- Hess J, Angel P, Schorpp-Kistner M. AP-1 subunits: quarrel and harmony among siblings. J Cell Sci. 2004;117:5965–5973. - PubMed
-
- Shaulian E, Karin M. AP-1 in cell proliferation and survival. Oncogene. 2001;20:2390–2400. - PubMed
-
- Eferl R, Wagner EF. AP-1: a double-edged sword in tumorigenesis. Nat Rev Cancer. 2003;3:859–868. - PubMed
-
- Karin M, Gallagher E. From JNK to pay dirt: Jun Kinases, their Biochemistry, physiology and clinical importance. IUBMB Life. 2005;57:283–295. - PubMed
-
- Davis RJ. Signal Transduction by the JNK Group of MAP Kinases. Cell. 2000;103:239–252. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

