Lysyl oxidase activity regulates oncogenic stress response and tumorigenesis
- PMID: 24113189
- PMCID: PMC3824691
- DOI: 10.1038/cddis.2013.382
Lysyl oxidase activity regulates oncogenic stress response and tumorigenesis
Abstract
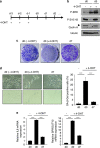
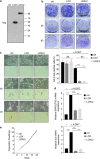
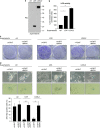
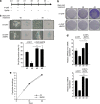
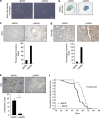
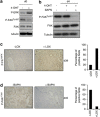
Cellular senescence, a stable proliferation arrest, is induced in response to various stresses. Oncogenic stress-induced senescence (OIS) results in blocked proliferation and constitutes a fail-safe program counteracting tumorigenesis. The events that enable a tumor in a benign senescent state to escape from OIS and become malignant are largely unknown. We show that lysyl oxidase activity contributes to the decision to maintain senescence. Indeed, in human epithelial cell the constitutive expression of the LOX or LOXL2 protein favored OIS escape, whereas inhibition of lysyl oxidase activity was found to stabilize OIS. The relevance of these in vitro observations is supported by in vivo findings: in a transgenic mouse model of aggressive pancreatic ductal adenocarcinoma (PDAC), increasing lysyl oxidase activity accelerates senescence escape, whereas inhibition of lysyl oxidase activity was found to stabilize senescence, delay tumorigenesis, and increase survival. Mechanistically, we show that lysyl oxidase activity favors the escape of senescence by regulating the focal-adhesion kinase. Altogether, our results demonstrate that lysyl oxidase activity participates in primary tumor growth by directly impacting the senescence stability.
Figures


Similar articles
-
Evolving roles of lysyl oxidase family in tumorigenesis and cancer therapy.Pharmacol Ther. 2020 Nov;215:107633. doi: 10.1016/j.pharmthera.2020.107633. Epub 2020 Jul 18. Pharmacol Ther. 2020. PMID: 32693113 Review.
-
Intracellular lysyl oxidase: effect of a specific inhibitor on nuclear mass in proliferating cells.Biochem Biophys Res Commun. 2010 Jun 11;396(4):944-9. doi: 10.1016/j.bbrc.2010.05.028. Epub 2010 May 9. Biochem Biophys Res Commun. 2010. PMID: 20460108
-
Stromally derived lysyl oxidase promotes metastasis of transforming growth factor-β-deficient mouse mammary carcinomas.Cancer Res. 2013 Sep 1;73(17):5336-46. doi: 10.1158/0008-5472.CAN-13-0012. Epub 2013 Jul 15. Cancer Res. 2013. PMID: 23856251 Free PMC article.
-
Human breast cancer cell metastasis is attenuated by lysyl oxidase inhibitors through down-regulation of focal adhesion kinase and the paxillin-signaling pathway.Breast Cancer Res Treat. 2012 Aug;134(3):989-1004. doi: 10.1007/s10549-012-1986-8. Epub 2012 Mar 21. Breast Cancer Res Treat. 2012. PMID: 22434522
-
Lysyl Oxidase and the Tumor Microenvironment.Int J Mol Sci. 2016 Dec 29;18(1):62. doi: 10.3390/ijms18010062. Int J Mol Sci. 2016. PMID: 28036074 Free PMC article. Review.
Cited by
-
ΔNp63 targets cytoglobin to inhibit oxidative stress-induced apoptosis in keratinocytes and lung cancer.Oncogene. 2016 Mar 24;35(12):1493-503. doi: 10.1038/onc.2015.222. Epub 2015 Jun 22. Oncogene. 2016. PMID: 26096935
-
LOX expression and functional analysis in astrocytomas and impact of IDH1 mutation.PLoS One. 2015 Mar 19;10(3):e0119781. doi: 10.1371/journal.pone.0119781. eCollection 2015. PLoS One. 2015. PMID: 25790191 Free PMC article.
-
Targeting the LOX/hypoxia axis reverses many of the features that make pancreatic cancer deadly: inhibition of LOX abrogates metastasis and enhances drug efficacy.EMBO Mol Med. 2015 Aug;7(8):1063-76. doi: 10.15252/emmm.201404827. EMBO Mol Med. 2015. PMID: 26077591 Free PMC article.
-
Inhibition of Copper Transport Induces Apoptosis in Triple-Negative Breast Cancer Cells and Suppresses Tumor Angiogenesis.Mol Cancer Ther. 2019 May;18(5):873-885. doi: 10.1158/1535-7163.MCT-18-0667. Epub 2019 Mar 1. Mol Cancer Ther. 2019. PMID: 30824611 Free PMC article.
-
Expression of the Calcium-Binding Protein CALB1 Is Induced and Controls Intracellular Ca2+ Levels in Senescent Cells.Int J Mol Sci. 2022 Aug 19;23(16):9376. doi: 10.3390/ijms23169376. Int J Mol Sci. 2022. PMID: 36012633 Free PMC article.
References
-
- Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. - PubMed
-
- Collado M, Gil J, Efeyan A, Guerra C, Schuhmacher AJ, Barradas M, et al. Tumour biology: Senescence in premalignant tumours. Nature. 2005;436:642. - PubMed
-
- Michaloglou C, Vredeveld LC, Soengas MS, Denoyelle C, Kuilman T, van der Horst CM, et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436:720–724. - PubMed
-
- Acosta JC, O'Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, et al. Chemokine Signaling via the CXCR2 Receptor Reinforces Senescence. Cell. 2008;133:1006–1018. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

