Classification of rhabdomyosarcoma and its molecular basis
- PMID: 24113309
- PMCID: PMC6637949
- DOI: 10.1097/PAP.0b013e3182a92d0d
Classification of rhabdomyosarcoma and its molecular basis
Abstract
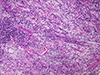
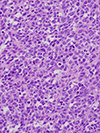
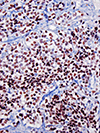
Rhabdomyosarcoma (RMS), the most common soft tissue sarcoma in children, has traditionally been classified into embryonal rhabdomyosarcoma (ERMS) and alveolar rhabdomyosarcoma (ARMS) for pediatric oncology practice. This review outlines the historical development of classification of childhood RMS and the challenges that have been associated with it, particularly problems with the diagnosis of "solid variant" ARMS and its distinction from ERMS. In addition to differences in clinical presentation and outcome, a number of genetic features underpin separation of ERMS from ARMS. Genetic differences associated with RMS subclassification include the presence of reciprocal translocations and their associated fusions in ARMS, amplification of genes in ARMS and its fusion subsets, chromosomal losses and gains that mostly occur in ERMS, and allelic losses and mutations usually associated with ERMS. Chimeric proteins encoded in most ARMS from the fusion of PAX3 or PAX7 with FOXO1 are expressed, result in a distinct pattern of downstream protein expression, and appear to be the proximate cause of the bad outcome associated with this subtype. A sizeable minority of ARMS lacks these fusions and shares the clinical and biological features of ERMS. A battery of immunohistochemical tests may prove useful in separating ERMS from ARMS and fusion-positive ARMS from fusion-negative ARMS. Because of limitation of predicting outcome solely based on histologic classification, treatment protocols will begin to utilize fusion testing for stratification of affected patients into low-risk, intermediate-risk, and high-risk groups.
Figures









References
-
- Parham DM, Alaggio R, Coffin CM. Myogenic tumors in children and adolescents. Pediatr Dev Pathol. 2012;15:211–238. - PubMed
-
- Kodet R, Fajstavr J, Kabelka Z, et al. Is fetal cellular rhabdomyoma an entity or a differentiated rhabdomyosarcoma? A study of patients with rhabdomyoma of the tongue and sarcoma of the tongue enrolled in the intergroup rhabdomyosarcoma studies I, II, and III. Cancer. 1991;67:2907–2913. - PubMed
-
- Cessna MH, Zhou H, Perkins SL, et al. Are myogenin and myoD1 expression specific for rhabdomyosarcoma? A study of 150 cases, with emphasis on spindle cell mimics. Am J Surg Pathol. 2001;25:1150–1157. - PubMed
-
- Folpe AL. MyoD1 and myogenin expression in human neoplasia: a review and update. Adv Anat Pathol. 2002;9:198–203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

