Hyperglycemia impairs atherosclerosis regression in mice
- PMID: 24113453
- PMCID: PMC5762940
- DOI: 10.1016/j.ajpath.2013.08.019
Hyperglycemia impairs atherosclerosis regression in mice
Abstract
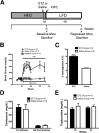
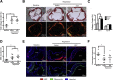
Diabetic patients are known to be more susceptible to atherosclerosis and its associated cardiovascular complications. However, the effects of hyperglycemia on atherosclerosis regression remain unclear. We hypothesized that hyperglycemia impairs atherosclerosis regression by modulating the biological function of lesional macrophages. HypoE (Apoe(h/h)Mx1-Cre) mice express low levels of apolipoprotein E (apoE) and develop atherosclerosis when fed a high-fat diet. Atherosclerosis regression occurs in these mice upon plasma lipid lowering induced by a change in diet and the restoration of apoE expression. We examined the morphological characteristics of regressed lesions and assessed the biological function of lesional macrophages isolated with laser-capture microdissection in euglycemic and hyperglycemic HypoE mice. Hyperglycemia induced by streptozotocin treatment impaired lesion size reduction (36% versus 14%) and lipid loss (38% versus 26%) after the reversal of hyperlipidemia. However, decreases in lesional macrophage content and remodeling in both groups of mice were similar. Gene expression analysis revealed that hyperglycemia impaired cholesterol transport by modulating ATP-binding cassette A1, ATP-binding cassette G1, scavenger receptor class B family member (CD36), scavenger receptor class B1, and wound healing pathways in lesional macrophages during atherosclerosis regression. Hyperglycemia impairs both reduction in size and loss of lipids from atherosclerotic lesions upon plasma lipid lowering without significantly affecting the remodeling of the vascular wall.
Copyright © 2013 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Shanmugam N., Román-Rego A., Ong P., Kaski J.C. Atherosclerotic plaque regression: fact or fiction? Cardiovasc Drugs Ther. 2010;24:311–317. - PubMed
-
- Williams K.J. Lipoprotein retention–and clues for atheroma regression. Arterioscler Thromb Vasc Biol. 2005;25:1536–1540. - PubMed
-
- Armstrong M.L., Megan M.B. Lipid depletion in atheromatous coronary arteries in rhesus monkeys after regression diets. Circ Res. 1972;30:675–680. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

