Septins promote dendrite and axon development by negatively regulating microtubule stability via HDAC6-mediated deacetylation
- PMID: 24113571
- PMCID: PMC3826633
- DOI: 10.1038/ncomms3532
Septins promote dendrite and axon development by negatively regulating microtubule stability via HDAC6-mediated deacetylation
Abstract
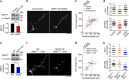
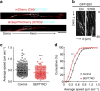
Neurite growth requires two guanine nucleotide-binding protein polymers of tubulins and septins. However, whether and how those cytoskeletal systems are coordinated was unknown. Here we show that the acute knockdown or knockout of the pivotal septin subunit SEPT7 from cerebrocortical neurons impairs their interhemispheric and cerebrospinal axon projections and dendritogenesis in perinatal mice, when the microtubules are severely hyperacetylated. The resulting hyperstabilization and growth retardation of microtubules are demonstrated in vitro. The phenotypic similarity between SEPT7 depletion and the pharmacological inhibition of α-tubulin deacetylase HDAC6 reveals that HDAC6 requires SEPT7 not for its enzymatic activity, but to associate with acetylated α-tubulin. These and other findings indicate that septins provide a physical scaffold for HDAC6 to achieve efficient microtubule deacetylation, thereby negatively regulating microtubule stability to an optimal level for neuritogenesis. Our findings shed light on the mechanisms underlying the HDAC6-mediated coupling of the two ubiquitous cytoskeletal systems during neural development.
Figures





References
-
- Mitchison T. & Kirschner M. Cytoskeletal dynamics and nerve growth. Neuron 1, 761–772 (1988). - PubMed
-
- Conde C. & Caceres A. Microtubule assembly, organization and dynamics in axons and dendrites. Nat. Rev. Neurosci. 10, 319–332 (2009). - PubMed
-
- Sulston J. E. & Horvitz H. R. Abnormal cell lineages in mutants of the nematode Caenorhabditis elegans. Dev. Biol. 82, 41–55 (1981). - PubMed
-
- White J. G., Horvitz H. R. & Sulston J. E. Neurone differentiation in cell lineage mutants of Caenorhabditis elegans. Nature 297, 584–587 (1982). - PubMed
-
- Sakamoto T. et al. Mass spectrometric analysis of microtubule co-sedimented proteins from rat brain. Genes Cells 13, 295–312 (2008). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

