Cbl ubiquitination of p85 is essential for Epo-induced EpoR endocytosis
- PMID: 24113870
- PMCID: PMC3854114
- DOI: 10.1182/blood-2013-05-506212
Cbl ubiquitination of p85 is essential for Epo-induced EpoR endocytosis
Abstract
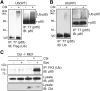
Erythropoietin (Epo) binding to the Epo receptor (EpoR) elicits downstream signaling that is essential for red blood cell production. One important negative regulatory mechanism to terminate Epo signaling is Epo-induced EpoR endocytosis and degradation. Defects in this mechanism play a key role in the overproduction of erythrocytes in primary familial and congenital polycythemia (PFCP). Here we have identified a novel mechanism mediating Epo-dependent EpoR internalization. Epo induces Cbl-dependent ubiquitination of the p85 regulatory subunit of PI3K, which binds to phosphotyrosines on EpoR. Ubiquitination allows p85 to interact with the endocytic protein epsin-1, thereby driving EpoR endocytosis. Knockdown of Cbl, expression of its dominant negative forms, or expression of an epsin-1 mutant devoid of ubiquitin-interacting motifs all compromise Epo-induced EpoR internalization. Mutated EpoRs mimicking those from PFCP patients cannot bind p85, co-localize with epsin-1, or internalize on Epo stimulation and exhibit Epo hypersensitivity. Similarly, knockdown of Cbl also causes Epo hypersensitivity in primary erythroid progenitors. Restoring p85 binding to PFCP receptors rescues Epo-induced epsin-1 co-localization and EpoR internalization and normalizes Epo hypersensitivity. Our results uncover a novel Cbl/p85/epsin-1 pathway in EpoR endocytosis and show that defects in this pathway contribute to excessive Epo signaling and erythroid hyperproliferation in PFCP.
Figures






References
-
- Constantinescu SN, Ghaffari S, Lodish HF. The Erythropoietin Receptor: Structure, Activation and Intracellular Signal Transduction. Trends Endocrinol Metab. 1999;10(1):18–23. - PubMed
-
- Richmond TD, Chohan M, Barber DL. Turning cells red: signal transduction mediated by erythropoietin. Trends Cell Biol. 2005;15(3):146–155. - PubMed
-
- Levine RL. JAK-mutant myeloproliferative neoplasms. Curr Top Microbiol Immunol. 2012;355:119–133. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

