LRRK2 phosphorylates novel tau epitopes and promotes tauopathy
- PMID: 24113872
- PMCID: PMC3830748
- DOI: 10.1007/s00401-013-1188-4
LRRK2 phosphorylates novel tau epitopes and promotes tauopathy
Abstract
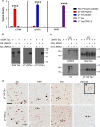
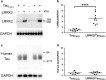
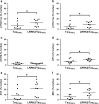
Mutations in the gene encoding leucine-rich repeat kinase 2 (LRRK2) are the most frequent cause of familial Parkinson's disease (PD). The neuropathology of LRRK2-related PD is heterogeneous and can include aberrant tau phosphorylation or neurofibrillary tau pathology. Recently, LRRK2 has been shown to phosphorylate tau in vitro; however, the major epitopes phosphorylated by LRRK2 and the physiological or pathogenic consequences of these modifications in vivo are unknown. Using mass spectrometry, we identified multiple sites on recombinant tau that are phosphorylated by LRRK2 in vitro, including pT149 and pT153, which are phospho-epitopes that to date have been largely unexplored. Importantly, we demonstrate that expression of transgenic LRRK2 in a mouse model of tauopathy increased the aggregation of insoluble tau and its phosphorylation at T149, T153, T205, and S199/S202/T205 epitopes. These findings indicate that tau can be a LRRK2 substrate and that this interaction can enhance salient features of human disease.
Figures




References
-
- Anand VS, Reichling LJ, Lipinski K, Stochaj W, Duan W, Kelleher K, Pungaliya P, Brown EL, Reinhart PH, Somberg R, Hirst WD, Riddle SM, Braithwaite SP. Investigation of leucine-rich repeat kinase 2: enzymological properties and novel assays. FEBS J. 2009;276(2):466–478. doi: 10.1111/j.1742-4658.2008.06789.x. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

