Optimal time for initiating antiretroviral therapy (ART) in HIV-infected, treatment-naive children aged 2 to 5 years old
- PMID: 24114324
- PMCID: PMC3950819
- DOI: 10.1002/14651858.CD010309.pub2
Optimal time for initiating antiretroviral therapy (ART) in HIV-infected, treatment-naive children aged 2 to 5 years old
Abstract
Background: The use of combination antiretroviral therapy (cART) comprising three antiretroviral medications from at least two classes of drugs is the current standard treatment for HIV infection in adults and children. Current World Health Organization (WHO) guidelines for antiretroviral therapy recommend early treatment regardless of immunologic thresholds or the clinical condition for all infants (less than one years of age) and children under the age of two years. For children aged two to five years current WHO guidelines recommend (based on low quality evidence) that clinical and immunological thresholds be used to identify those who need to start cART (advanced clinical stage or CD4 counts ≤ 750 cells/mm(3) or per cent CD4 ≤ 25%). This Cochrane review will inform the current available evidence regarding the optimal time for treatment initiation in children aged two to five years with the goal of informing the revision of WHO 2013 recommendations on when to initiate cART in children.
Objectives: To assess the evidence for the optimal time to initiate cART in treatment-naive, HIV-infected children aged 2 to 5 years.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, the AEGIS conference database, specific relevant conferences, www.clinicaltrials.gov, the World Health Organization International Clinical Trials Registry platform and reference lists of articles. The date of the most recent search was 30 September 2012.
Selection criteria: Randomised controlled trials (RCTs) that compared immediate with deferred initiation of cART, and prospective cohort studies which followed children from enrolment to start of cART and on cART.
Data collection and analysis: Two review authors considered studies for inclusion in the review, assessed the risk of bias, and extracted data on the primary outcome of death from all causes and several secondary outcomes, including incidence of CDC category C and B clinical events and per cent CD4 cells (CD4%) at study end. For RCTs we calculated relative risks (RR) or mean differences with 95% confidence intervals (95% CI). For cohort data, we extracted relative risks with 95% CI from adjusted analyses. We combined results from RCTs using a random effects model and examined statistical heterogeneity.
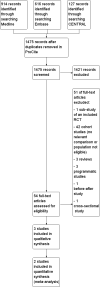
Main results: Two RCTs in HIV-positive children aged 1 to 12 years were identified. One trial was the pilot study for the larger second trial and both compared initiation of cART regardless of clinical-immunological conditions with deferred initiation until per cent CD4 dropped to <15%. The two trials were conducted in Thailand, and Thailand and Cambodia, respectively. Unpublished analyses of the 122 children enrolled at ages 2 to 5 years were included in this review. There was one death in the immediate cART group and no deaths in the deferred group (RR 2.9; 95% CI 0.12 to 68.9). In the subgroup analysis of children aged 24 to 59 months, there was one CDC C event in each group (RR 0.96; 95% CI 0.06 to 14.87) and 8 and 11 CDC B events in the immediate and deferred groups respectively (RR 0.95; 95% CI 0.24 to 3.73). In this subgroup, the mean difference in CD4 per cent at study end was 5.9% (95% CI 2.7 to 9.1). One cohort study from South Africa, which compared the effect of delaying cART for up to 60 days in 573 HIV-positive children starting tuberculosis treatment (median age 3.5 years), was also included. The adjusted hazard ratios for the effect on mortality of delaying ART for more than 60 days was 1.32 (95% CI 0.55 to 3.16).
Authors' conclusions: This systematic review shows that there is insufficient evidence from clinical trials in support of either early or CD4-guided initiation of ART in HIV-infected children aged 2 to 5 years. Programmatic issues such as the retention in care of children in ART programmes in resource-limited settings will need to be considered when formulating WHO 2013 recommendations.
Conflict of interest statement
NS has no conflict of interest. ME and MD are investigators in the collaborative IeDEA cohort studies which evaluate the effects of cART on outcomes in HIV‐infected children. MP is a consultant for the WHO and LM is an employee of the WHO.
Figures





























Update of
- doi: 10.1002/14651858.CD010309
References
References to studies included in this review
Ananworanich 2008 {published data only}
PREDICT 2012 {published and unpublished data}
-
- Wongsawat J, Puthanakit T, Kanjanavanit S, Hansudewechakul R, Ngampiyaskul C, Kerr SJ, et al. CD4 cell count criteria to determine when to initiate antiretroviral therapy in human immunodeficiency virus‐infected children. The Pediatric infectious disease journal 2010;29(10):966‐8. [PUBMED: 20418798] - PMC - PubMed
Yotebieng 2010 {published data only}
References to studies excluded from this review
Edmonds 2009 {published data only}
Edmonds 2011 {published data only}
Munyagwa 2012 {published data only}
-
- Munyagwa M, Baisley K, Levin J, Brian M, Grosskurth H, Maher D. Mortality of HIV‐infected and uninfected children in a longitudinal cohort in rural south‐west Uganda during 8 years of follow‐up. Tropical medicine & international health : TM & IH 2012;17(7):836‐43. [PUBMED: 22591447] - PubMed
Musoke 2010 {published data only}
Patel 2008 a {published data only}
-
- Patel K, Hernán MA, Williams PL, Seeger JD, McIntosh K, Dyke RB, Seage GR 3rd, Pediatric AIDS Clinical Trials Group 219/219C Study Team. Long‐term effects of highly active antiretroviral therapy on CD4+ cell evolution among children and adolescents infected with HIV: 5 years and counting. Clin Infect Dis 2008;46(11):1751‐60. [PUBMED: 18426371] - PMC - PubMed
Patel 2008 b {published data only}
-
- Patel K, Hernan MA, Williams PL, Seeger JD, McIntosh K, Dyke RB, Seage GR 3rd, Pediatric AIDS Clinical Trials Group 219/219C Study Team. Long‐term effectiveness of highly active antiretroviral therapy on the survival of children and adolescents with HIV infection: a 10‐year follow‐up study. Clinical infectious Diseases 2008;46(4):507‐15. [PUBMED: 18199042] - PubMed
Additional references
DAIDS 2009
-
- Division of AIDS. Table for grading the severity of Adult and Pediatric Adverse Events. Accessed 28 October 2009 http://rcc.tech‐res.com/Document/safetyandpharmacovigilance/DAIDS_AE_GradingTable_Clarif....
Fenner 2010
-
- Fenner L, Brinkhof MW, Keiser O, Weigel R, Cornell M, Moultrie H, Prozesky H, Technau K, Eley B, Vaz P, Pascoe M, Giddy J, Cutsem G, Wood R, Egger M, Davies MA. Early mortality and loss to follow‐up in HIV‐infected children starting antiretroviral therapy in Southern Africa. J Acquir Immune Defic Syndr 2010;54(5):542‐532. - PMC - PubMed
GradePro 2008 [Computer program]
-
- Jan Bozek, Andrew Oxman, Olger Schunemann. GradePro 2008. Version 3.2 for Windows. GRADE Working Group, 2008, 2008.
Guyatt 2011
-
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of clinical epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] - PubMed
Heidari 2012
Higgins 2002
-
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. - PubMed
Higgins 2009
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane Collaboration, 2009. Available from www.cochrane‐handbook.org.
Lewis 2011
-
- Lewis J, Walker AS, Castro H, Rossi A, Gibb DM, Giaquinto C, Klein N, Callard R. Age and CD4 Count at Initiation of Antiretroviral Therapy in HIV‐Infected Children: Effects on Long‐term T‐Cell Reconstitution. J Infect Dis 2011;205:548‐56. - PubMed
Patel 2008
-
- Patel K, Hernan MA, Williams PL, Seeger JD, McIntosh K, Dyke RB, et al. Long‐term effectiveness of highly active antiretroviral therapy on the survival of children and adolescents with HIV infection: a 10‐year follow‐up study. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2008;46(4):507‐15. [PUBMED: 18199042] - PubMed
Penazzato 2012
-
- Penazzato M, Prendergast A, Tierney J, Cotton M, Gibb D. Effectiveness of antiretroviral therapy in HIV‐infected children under 2 years of age. Cochrane database of systematic reviews (Online) 2012;7:CD004772. [PUBMED: 22786492] - PubMed
Prendergast 2012
Robins 2000
-
- Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology 2000;11(5):550–560. - PubMed
UNAIDS 2012
-
- Joint United Nations Programme on HIV/AIDS (UNAIDS). Global report: UNAIDS report on the global AIDS epidemic 2012. http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiolo... 2012;Accessed 24 May 2013.
Violari 2008
WHO 2002
-
- World Health Organization. Scaling up antiretroviral therapy In resource‐limited settings: guidelines for a public health approach. 2002; Vol. http://www.who.int/hiv/pub/prev_care/en/ScalingUp_E.pdf (Accessed 29 July 2012).
WHO 2003
-
- World Health Organization. Scaling up antiretroviral therapy in resource‐limited settings:treatment guidelines for a public health approach [2003 revision]. 2003; Vol. http://www.who.int/hiv/pub/prev_care/en/arvrevision2003en.pdf (Accessed 29 July 2012).
WHO 2006a
-
- World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach [2006 revision]. World Health Organization, 2006; Vol. http://www.who.int/hiv/pub/guidelines/artadultguidelines.pdf (Accessed 29 July 2012). - PubMed
WHO 2006b
-
- World Health Organization. Antiretroviral therapy of HIV infection in infants and children: towards universal access. Recommendations for a public health approach. 2006; Vol. http://www.who.int/hiv/pub/guidelines/paediatric020907.pdf (Accessed 29 July 2012). - PubMed
WHO 2010a
-
- World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach [2010 revision]. 2010; Vol. http://whqlibdoc.who.int/publications/2010/9789241599764_eng.pdf (Accessed 29 July 2012). - PubMed
WHO 2010b
-
- World Health Organization. Antiretroviral therapy of HIV infection in infants and children: towards universal access: recommendations for a public health approach [2010 revision]. 2010; Vol. http://whqlibdoc.who.int/publications/2010/9789241599801_eng.pdf (Accessed 29 July 2012). - PubMed
WHO 2011a
-
- World Health Organization. Global HIV/AIDS response: epidemic update and health sector progress towards universal access: progress report 2011. 2011; Vol. http://whqlibdoc.who.int/publications/2011/9789241502986_eng.pdf (Accessed 2 August 2012).
WHO 2011b
-
- World Health Organization. The treatment 2.0 framework for action: catalysing the next phase of treatment, care and support. 2011; Vol. http://whqlibdoc.who.int/publications/2011/9789241501934_eng.pdf (Accessed 2 August 2012).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

