ENerGetIcs in hypertrophic cardiomyopathy: traNslation between MRI, PET and cardiac myofilament function (ENGINE study)
- PMID: 24114686
- PMCID: PMC3833912
- DOI: 10.1007/s12471-013-0478-8
ENerGetIcs in hypertrophic cardiomyopathy: traNslation between MRI, PET and cardiac myofilament function (ENGINE study)
Abstract
Introduction: Hypertrophic cardiomyopathy (HCM) is an autosomal dominant heart disease mostly due to mutations in genes encoding sarcomeric proteins. HCM is characterised by asymmetric hypertrophy of the left ventricle (LV) in the absence of another cardiac or systemic disease. At present it lacks specific treatment to prevent or reverse cardiac dysfunction and hypertrophy in mutation carriers and HCM patients. Previous studies have indicated that sarcomere mutations increase energetic costs of cardiac contraction and cause myocardial dysfunction and hypertrophy. By using a translational approach, we aim to determine to what extent disturbances of myocardial energy metabolism underlie disease progression in HCM.
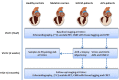
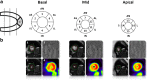
Methods: Hypertrophic obstructive cardiomyopathy (HOCM) patients and aortic valve stenosis (AVS) patients will undergo a positron emission tomography (PET) with acetate and cardiovascular magnetic resonance imaging (CMR) with tissue tagging before and 4 months after myectomy surgery or aortic valve replacement + septal biopsy. Myectomy tissue or septal biopsy will be used to determine efficiency of sarcomere contraction in-vitro, and results will be compared with in-vivo cardiac performance. Healthy subjects and non-hypertrophic HCM mutation carriers will serve as a control group.
Endpoints: Our study will reveal whether perturbations in cardiac energetics deteriorate during disease progression in HCM and whether these changes are attributed to cardiac remodelling or the presence of a sarcomere mutation per se. In-vitro studies in hypertrophied cardiac muscle from HOCM and AVS patients will establish whether sarcomere mutations increase ATP consumption of sarcomeres in human myocardium. Our follow-up imaging study in HOCM and AVS patients will reveal whether impaired cardiac energetics are restored by cardiac surgery.
Figures
References
-
- Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA. 2002;287:1308–1320. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

