The succinated proteome
- PMID: 24115015
- PMCID: PMC4038156
- DOI: 10.1002/mas.21382
The succinated proteome
Abstract
The post-translational modifications (PTMs) of cysteine residues include oxidation, S-glutathionylation, S-nitrosylation, and succination, all of which modify protein function or turnover in response to a changing intracellular redox environment. Succination is a chemical modification of cysteine in proteins by the Krebs cycle intermediate, fumarate, yielding S-(2-succino)cysteine (2SC). Intracellular fumarate concentration and succination of proteins are increased by hyperpolarization of the inner mitochondrial membrane, in concert with mitochondrial, endoplasmic reticulum (ER) and oxidative stress in 3T3 adipocytes grown in high glucose medium and in adipose tissue in obesity and diabetes in mice. Increased succination of proteins is also detected in the kidney of a fumarase deficient conditional knock-out mouse which develops renal cysts. A wide range of proteins are subject to succination, including enzymes, adipokines, cytoskeletal proteins, and ER chaperones with functional cysteine residues. There is also some overlap between succinated and glutathionylated proteins, suggesting that the same low pKa thiols are targeted by both. Succination of adipocyte proteins in diabetes increases as a result of nutrient excess derived mitochondrial stress and this is inhibited by uncouplers, which discharge the mitochondrial membrane potential (ΔΨm) and relieve the electron transport chain. 2SC therefore serves as a biomarker of mitochondrial stress or dysfunction in chronic diseases, such as obesity, diabetes, and cancer, and recent studies suggest that succination is a mechanistic link between mitochondrial dysfunction, oxidative and ER stress, and cellular progression toward apoptosis. In this article, we review the history of the succinated proteome and the challenges associated with measuring this non-enzymatic PTM of proteins by proteomics approaches.
Keywords: chemical modification of proteins; cysteine; diabetes; fumarate; succination.
© 2013 Wiley Periodicals, Inc.
Figures
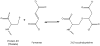




References
-
- Adam J, Hatipoglu E, O’Flaherty L, Ternette N, Sahgal N, Lockstone H, Baban D, Nye E, Stamp GW, Wolhuter K, Stevens M, Fischer R, Carmeliet P, Maxwell PH, Pugh CW, Frizzell N, Soga T, Kessler BM, El-Bahrawy M, Ratcliffe PJ, Pollard PJ. Renal cyst formation in Fh1-deficient mice is independent of the Hif/Phd pathway: Roles for fumarate in Keap1 succination and Nrf2 signaling. Cancer Cell. 2011;20:524–537. - PMC - PubMed
-
- Alderson NL, Wang YP, Blatnik M, Frizzell N, Walla MD, Lyons TJ, Alt N, Carson JA, Nagai R, Thorpe SR, Baynes JW. S-(2-succinyl) cysteine: A novel chemical modification of tissue proteins by a krebs cycle intermediate. Arch Biochem Biophys. 2006;450:1–8. - PubMed
-
- Alt N, Carson JA, Alderson NL, Wang YP, Nagai R, Henle T, Thorpe SR, Baynes JW. Chemical modification of muscle protein in diabetes. Arch Biochem Biophys. 2004;425:200–206. - PubMed
-
- Ashrafian H, Czibik G, Bellahcene M, Aksentijevic D, Smith AC, Mitchell SJ, Dodd MS, Kirwan J, Byrne JJ, Ludwig C, Isackson H, Yavari A, Stottrup NB, Contractor H, Cahill TJ, Sahgal N, Ball DR, Birkler RID, Hargreaves L, Tennant DA, Land J, Lygate CA, Johannsen M, Kharbanda RK, Neubauer S, Redwood C, de Cabo R, Ahmet I, Talan M, Gunther UL, Robinson AJ, Viant MR, Pollard PJ, Tyler DJ, Watkins H. Fumarate is cardioprotective via activation of the Nrf2 antioxidant pathway. Cell Metab. 2012;15:361–371. - PMC - PubMed
-
- Bardella C, El-Bahrawy M, Frizzell N, Adam J, Ternette N, Hatipoglu E, Howarth K, O’Flaherty L, Roberts I, Turner G, Taylor J, Giaslakiotis K, Macaulay VM, Harris AL, Chandra A, Lehtonen HJ, Launonen V, Aaltonen LA, Pugh CW, Mihai R, Trudgian D, Kessler B, Baynes JW, Ratcliffe PJ, Tomlinson IP, Pollard PJ. Aberrant succination of proteins in fumarate hydratase-deficient mice and hlrcc patients is a robust biomarker of mutation status. J Pathol. 2011;225:4–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

