Preclinical safety and activity of recombinant VSV-IFN-β in an immunocompetent model of squamous cell carcinoma of the head and neck
- PMID: 24115092
- PMCID: PMC3969865
- DOI: 10.1002/hed.23502
Preclinical safety and activity of recombinant VSV-IFN-β in an immunocompetent model of squamous cell carcinoma of the head and neck
Abstract
Background: Recombinant vesicular stomatitis virus expressing interferon-β (VSV-IFN-β) has demonstrated antitumor activity in vitro and in vivo. In preparation for clinical testing in human squamous cell carcinoma (SCC) of the head and neck, we conducted preclinical studies of VSV-IFN-β in syngeneic SCC models.
Methods: In vitro, VSV-IFN-β (expressing rat or mouse interferon [IFN]-β)-induced cytotoxicity and propagated in rat (FAT-7) or mouse (SCC-VII) SCC cells during normoxia and hypoxia. In vivo, intratumoral administration of VSV-rat-IFN-β or VSV-human-IFN-β in FAT-7 bearing or non-tumor bearing immunocompetent rats did not result in acute organ toxicity or death.
Results: VSV-r-IFN-β replicated predominantly in tumors and a dose dependent anti-VSV antibody response was observed. Intratumoral or intravenous administration of VSV-IFN-β resulted in growth delay and improved survival compared with controls.
Conclusion: The above data confirm safety and feasibility of VSV-IFN-β administration in immunocompetent animals and support its clinical evaluation in advanced human head and neck cancer.
Keywords: biodistribution; preclinical studies; squamous cell carcinoma; syngeneic models; vesicular stomatitis virus.
© 2014 Wiley Periodicals, Inc.
Figures
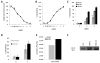
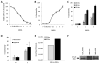
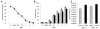
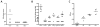
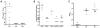

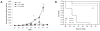
Similar articles
-
Preclinical efficacy of oncolytic VSV-IFNβ in treating cancer: A systematic review.Front Immunol. 2023 Mar 31;14:1085940. doi: 10.3389/fimmu.2023.1085940. eCollection 2023. Front Immunol. 2023. PMID: 37063914 Free PMC article.
-
Interferon Beta and Interferon Alpha 2a Differentially Protect Head and Neck Cancer Cells from Vesicular Stomatitis Virus-Induced Oncolysis.J Virol. 2015 Aug;89(15):7944-54. doi: 10.1128/JVI.00757-15. Epub 2015 May 20. J Virol. 2015. PMID: 25995245 Free PMC article.
-
Combined VSV oncolytic virus and chemotherapy for squamous cell carcinoma.Laryngoscope. 2008 Feb;118(2):237-42. doi: 10.1097/MLG.0b013e3181581977. Laryngoscope. 2008. PMID: 18043494
-
Oncolytic measles and vesicular stomatitis virotherapy for endometrial cancer.Gynecol Oncol. 2014 Jan;132(1):194-202. doi: 10.1016/j.ygyno.2013.11.010. Epub 2013 Nov 15. Gynecol Oncol. 2014. PMID: 24246772 Free PMC article.
-
Evaluation of an attenuated vesicular stomatitis virus vector expressing interferon-beta for use in malignant pleural mesothelioma: heterogeneity in interferon responsiveness defines potential efficacy.Hum Gene Ther. 2010 Jan;21(1):51-64. doi: 10.1089/hum.2009.088. Hum Gene Ther. 2010. PMID: 19715403 Free PMC article.
Cited by
-
Efficient Delivery and Replication of Oncolytic Virus for Successful Treatment of Head and Neck Cancer.Int J Mol Sci. 2020 Sep 25;21(19):7073. doi: 10.3390/ijms21197073. Int J Mol Sci. 2020. PMID: 32992948 Free PMC article. Review.
-
Immunomodulatory and antitumor effects of type I interferons and their application in cancer therapy.Oncotarget. 2017 Jul 25;8(41):71249-71284. doi: 10.18632/oncotarget.19531. eCollection 2017 Sep 19. Oncotarget. 2017. PMID: 29050360 Free PMC article. Review.
-
Preclinical efficacy of oncolytic VSV-IFNβ in treating cancer: A systematic review.Front Immunol. 2023 Mar 31;14:1085940. doi: 10.3389/fimmu.2023.1085940. eCollection 2023. Front Immunol. 2023. PMID: 37063914 Free PMC article.
-
Combination of IAP Antagonists and TNF-α-Armed Oncolytic Viruses Induce Tumor Vascular Shutdown and Tumor Regression.Mol Ther Oncolytics. 2018 Jun 21;10:28-39. doi: 10.1016/j.omto.2018.06.002. eCollection 2018 Sep 28. Mol Ther Oncolytics. 2018. PMID: 30101187 Free PMC article.
-
Interferon Beta and Interferon Alpha 2a Differentially Protect Head and Neck Cancer Cells from Vesicular Stomatitis Virus-Induced Oncolysis.J Virol. 2015 Aug;89(15):7944-54. doi: 10.1128/JVI.00757-15. Epub 2015 May 20. J Virol. 2015. PMID: 25995245 Free PMC article.
References
-
- Porosnicu M, Mian A, Barber GN. The oncolytic effect of recombinant vesicular stomatitis virus is enhanced by expression of the fusion cytosine deaminase/uracil phosphoribosyltransferase suicide gene. Cancer research. 2003;63(23):8366–76. - PubMed
-
- Haddad RI, Shin DM. Recent advances in head and neck cancer. N Engl J Med. 2008;359(11):1143–54. - PubMed
-
- Shin DM, Khuri FR. Advances in the management of recurrent or metastatic squamous cell carcinoma of the head and neck. Head Neck. 2011 - PubMed
-
- O’Rorke MA, Ellison MV, Murray LJ, Moran M, James J, Anderson LA. Human papillomavirus related head and neck cancer survival: A systematic review and meta-analysis. Oral Oncol. 2012 - PubMed
-
- de Andrade DA, Machiels JP. Treatment options for patients with recurrent or metastatic squamous cell carcinoma of the head and neck, who progress after platinum-based chemotherapy. Curr Opin Oncol. 2012;24(3):211–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

