The regulation and deregulation of Wnt signaling by PARK genes in health and disease
- PMID: 24115276
- PMCID: PMC4344548
- DOI: 10.1093/jmcb/mjt037
The regulation and deregulation of Wnt signaling by PARK genes in health and disease
Abstract
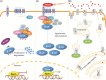
Wingless/Int (Wnt) signaling pathways are signal transduction mechanisms that have been widely studied in the field of embryogenesis. Recent work has established a critical role for these pathways in brain development, especially of midbrain dopaminergic neurones. However, the fundamental importance of Wnt signaling for the normal function of mature neurones in the adult central nervous system has also lately been demonstrated by an increasing number of studies. Parkinson's disease (PD) is the second most prevalent neurodegenerative disease worldwide and is currently incurable. This debilitating disease is characterized by the progressive loss of a subset of midbrain dopaminergic neurones in the substantia nigra leading to typical extrapyramidal motor symptoms. The aetiology of PD is poorly understood but work performed over the last two decades has identified a growing number of genetic defects that underlie this condition. Here we review a growing body of data connecting genes implicated in PD--most notably the PARK genes--with Wnt signaling. These observations provide clues to the normal function of these proteins in healthy neurones and suggest that deregulated Wnt signaling might be a frequent pathomechanism leading to PD. These observations have implications for the pathogenesis and treatment of neurodegenerative diseases in general.
Keywords: LRRK2; Parkinson's disease; Wnt signaling; genetics of Parkinson's disease; neurodegeneration; treatment.
Figures
References
-
- Arenas E. Engineering a dopaminergic phenotype in stem/precursor cells: role of Nurr1, glia-derived signals, and Wnts. Ann. NY Acad. Sci. 2005;1049:51–66. - PubMed
-
- Belenkaya T.Y., Wu Y., Tang X., et al. The retromer complex influences Wnt secretion by recycling wntless from endosomes to the trans-Golgi network. Dev. Cell. 2008;14:120–131. - PubMed
-
- Berwick D.C., Harvey K. LRRK2 signaling pathways: the key to unlocking neurodegeneration? Trends Cell Biol. 2011;21:257–265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

