Assessing optimal target populations for influenza vaccination programmes: an evidence synthesis and modelling study
- PMID: 24115913
- PMCID: PMC3793005
- DOI: 10.1371/journal.pmed.1001527
Assessing optimal target populations for influenza vaccination programmes: an evidence synthesis and modelling study
Abstract
Background: Influenza vaccine policies that maximise health benefit through efficient use of limited resources are needed. Generally, influenza vaccination programmes have targeted individuals 65 y and over and those at risk, according to World Health Organization recommendations. We developed methods to synthesise the multiplicity of surveillance datasets in order to evaluate how changing target populations in the seasonal vaccination programme would affect infection rate and mortality.
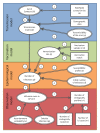
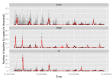
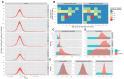
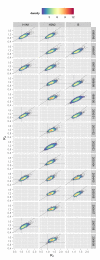
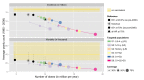
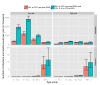
Methods and findings: Using a contemporary evidence-synthesis approach, we use virological, clinical, epidemiological, and behavioural data to develop an age- and risk-stratified transmission model that reproduces the strain-specific behaviour of influenza over 14 seasons in England and Wales, having accounted for the vaccination uptake over this period. We estimate the reduction in infections and deaths achieved by the historical programme compared with no vaccination, and the reduction had different policies been in place over the period. We find that the current programme has averted 0.39 (95% credible interval 0.34-0.45) infections per dose of vaccine and 1.74 (1.16-3.02) deaths per 1,000 doses. Targeting transmitters by extending the current programme to 5-16-y-old children would increase the efficiency of the total programme, resulting in an overall reduction of 0.70 (0.52-0.81) infections per dose and 1.95 (1.28-3.39) deaths per 1,000 doses. In comparison, choosing the next group most at risk (50-64-y-olds) would prevent only 0.43 (0.35-0.52) infections per dose and 1.77 (1.15-3.14) deaths per 1,000 doses.
Conclusions: This study proposes a framework to integrate influenza surveillance data into transmission models. Application to data from England and Wales confirms the role of children as key infection spreaders. The most efficient use of vaccine to reduce overall influenza morbidity and mortality is thus to target children in addition to older adults. Please see later in the article for the Editors' Summary.
Conflict of interest statement
ND is a member of the Editorial Board of PLOS Medicine where he acts as a statistical adviser.
Figures
References
-
- Mauskopf J, Klesse M, Lee S, Herrera-Taracena G (2012) The burden of influenza complications in different high-risk groups: a targeted literature review. J Med Econ 16: 264–277. - PubMed
-
- World Health Organization (2005) Influenza vaccines—WHO position paper. Wkly Epidemiol Rec 33: 279–287 Available: http://www.who.int/immunization/wer8033influenza_August2005_position_pap.... Accessed 5 September 2013.
-
- World Health Organization (2012) Vaccines against influenza—WHO position paper. Wkly Epidemiol Rec 87: 461–476. - PubMed
-
- Viboud C, Boëlle P-Y, Cauchemez S, Lavenu A, Valleron A-J, et al. (2004) Risk factors of influenza transmission in households. Br J Gen Pract 54: 684–689 Available: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1326070/. Accessed 5 September 2013. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

