Emerging role of the endoplasmic reticulum in peroxisome biogenesis
- PMID: 24115935
- PMCID: PMC3792350
- DOI: 10.3389/fphys.2013.00286
Emerging role of the endoplasmic reticulum in peroxisome biogenesis
Abstract
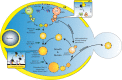
During the past few years, we have witnessed a paradigm shift in our long-standing concept of peroxisome biogenesis. Recent biochemical and morphological studies have revealed a primary role of the endoplasmic reticulum (ER) in the de novo formation of peroxisomes, thus challenging the prevalent model invoking growth and division of pre-existing peroxisomes. Importantly, a novel sorting process has been recently defined at the ER that segregates and assembles specific sets of peroxisomal membrane proteins (PMPs) into distinct pre-peroxisomal vesicular carriers (ppVs) that later undergo heterotypic fusion to form mature peroxisomes. Consequently, the emerging model has redefined the function of many peroxins (most notably Pex3, Pex19, and Pex25) and assigned them novel roles in vesicular budding and subsequent peroxisome assembly. These advances establish a novel intracellular membrane trafficking route between the ER and peroxisomes, but the components remain elusive. This review will provide a historical perspective and focus on recent developments in the emerging role of the ER in peroxisome biogenesis.
Keywords: ER involvement in peroxisome biogenesis; intracellular protein trafficking; organelle biogenesis; peroxisomal ER; peroxisome; vesicle budding.
Figures
References
-
- Baerends R. J., Rasmussen S. W., Hilbrands R. E., van der Heide M., Faber K. N., Reuvekamp P. T., et al. (1996). The Hansenula polymorpha PER9 gene encodes a peroxisomal membrane protein essential for peroxisome assembly and integrity. J. Biol. Chem. 271, 8887–8894 10.1074/jbc.271.15.8887 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

