Activated effects of parathyroid hormone-related protein on human hepatic stellate cells
- PMID: 24116114
- PMCID: PMC3792035
- DOI: 10.1371/journal.pone.0076517
Activated effects of parathyroid hormone-related protein on human hepatic stellate cells
Abstract
Background & aims: After years of experiments and clinical studies, parathyroid hormone-related protein(PTHrP) has been shown to be a bone formation promoter that elicits rapid effects with limited adverse reaction. Recently, PTHrP was reported to promote fibrosis in rat kidney in conjunction with transforming growth factor-beta1 (TGF-β1), which is also a fibrosis promoter in liver. However, the effect of PTHrP in liver has not been determined. In this study, the promoting actions of PTHrP were first investigated in human normal hepatic stellate cells (HSC) and LX-2 cell lines.
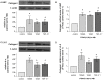
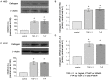
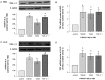
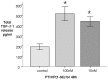
Methods: TGF-β1, alpha-smooth muscle actin (α-SMA), matrix metalloproteinase 2 (MMP-2), and collagen I mRNA were quantified by real-time polymerase chain reaction (PCR) after HSCs or LX-2 cells were treated with PTHrP(1-36) or TGF-β1. Protein levels were also assessed by western-blot analysis. Alpha-SMA were also detected by immunofluorescence, and TGF-β1 secretion was measured with enzyme-linked immunosorbent assay (ELISA) of HSC cell culture media.
Results: In cultured human HSCs, mRNA and protein levels of α-SMA, collagen I, MMP-2, and TGF-β1 were increased by PTHrP treatment. A similar increasing pattern was also observed in LX-2 cells. Moreover, PTHrP significantly increased TGF-β1 secretion in cultured media from HSCs.
Conclusions: PTHrP activated HSCs and promoted the fibrosis process in LX-2 cells. These procedures were probably mediated via TGF-β1, highlighting the potential effects of PTHrP in the liver.
Conflict of interest statement
Figures



Similar articles
-
Newcastle disease virus represses the activation of human hepatic stellate cells and reverses the development of hepatic fibrosis in mice.Liver Int. 2009 Apr;29(4):593-602. doi: 10.1111/j.1478-3231.2009.01971.x. Epub 2009 Jan 28. Liver Int. 2009. PMID: 19192169
-
Ethanol-stimulated differentiated functions of human or mouse hepatic stellate cells are mediated by connective tissue growth factor.J Hepatol. 2011 Aug;55(2):399-406. doi: 10.1016/j.jhep.2010.11.025. Epub 2010 Dec 13. J Hepatol. 2011. PMID: 21156189 Free PMC article.
-
Astaxanthin prevents TGFβ1-induced pro-fibrogenic gene expression by inhibiting Smad3 activation in hepatic stellate cells.Biochim Biophys Acta. 2015 Jan;1850(1):178-85. doi: 10.1016/j.bbagen.2014.10.014. Epub 2014 Oct 23. Biochim Biophys Acta. 2015. PMID: 25450180
-
Caffeic acid phenethyl ester attenuates liver fibrosis via inhibition of TGF-β1/Smad3 pathway and induction of autophagy pathway.Biochem Biophys Res Commun. 2017 Apr 22;486(1):22-28. doi: 10.1016/j.bbrc.2017.02.057. Epub 2017 Feb 11. Biochem Biophys Res Commun. 2017. PMID: 28193525
-
PM2.5-exposed hepatocytes induce hepatic stellate cells activation by releasing TGF-β1.Biochem Biophys Res Commun. 2021 Sep 10;569:125-131. doi: 10.1016/j.bbrc.2021.07.002. Epub 2021 Jul 7. Biochem Biophys Res Commun. 2021. PMID: 34243068
Cited by
-
Role of G Protein-Coupled Receptors in Hepatic Stellate Cells and Approaches to Anti-Fibrotic Treatment of Non-Alcoholic Fatty Liver Disease.Front Endocrinol (Lausanne). 2021 Dec 6;12:773432. doi: 10.3389/fendo.2021.773432. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34938271 Free PMC article. Review.
-
Metabolic Health and Disease: A Role of Osteokines?Calcif Tissue Int. 2023 Jul;113(1):21-38. doi: 10.1007/s00223-023-01093-0. Epub 2023 May 17. Calcif Tissue Int. 2023. PMID: 37193929 Review.
-
[Parathyroid hormone-related protein aggravates nonalcoholic fatty liver disease induced by methionine choline-deficient diet in mice].Nan Fang Yi Ke Da Xue Xue Bao. 2021 Jul 20;41(7):1037-1043. doi: 10.12122/j.issn.1673-4254.2021.07.10. Nan Fang Yi Ke Da Xue Xue Bao. 2021. PMID: 34308853 Free PMC article. Chinese.
-
Parathyroid Hormone Related Protein (PTHrP)-Associated Molecular Signatures in Tissue Differentiation and Non-Tumoral Diseases.Biology (Basel). 2023 Jul 3;12(7):950. doi: 10.3390/biology12070950. Biology (Basel). 2023. PMID: 37508381 Free PMC article. Review.
References
-
- Bisello A, Horwitz MJ, Stewart AF (2004) Parathyroid hormone-related protein: an essential physiological regulator of adult bone mass. Endocrinology 145: 3551–3553. - PubMed
-
- Horwitz MJ, Tedesco MB, Gundberg C, Garcia-Ocana A, Stewart AF (2003) Short-term, high-dose parathyroid hormone-related protein as a skeletal anabolic agent for the treatment of postmenopausal osteoporosis. J Clin Endocrinol Metab 88: 569–575. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

