Nevirapine-based regimens in HIV-infected antiretroviral-naive patients: systematic review and meta-analysis of randomized controlled trials
- PMID: 24116123
- PMCID: PMC3792044
- DOI: 10.1371/journal.pone.0076587
Nevirapine-based regimens in HIV-infected antiretroviral-naive patients: systematic review and meta-analysis of randomized controlled trials
Abstract
Background: Nevirapine belongs to the group of non-nucleoside reverse transcriptase inhibitors (NNRTIs) and is commonly administered in first-line treatment of HIV infection.
Objective: Systematic review and meta-analysis was undertaken to compare effectiveness of nevirapine-based regimens with other antiretroviral schedules used as an initial treatment of HIV-infected antiretroviral-naive subjects.
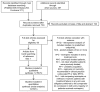
Methods: Electronic databases (PubMed, EMBASE, the Cochrane Library, Trip Database) were searched up to 28 December 2012 for randomized controlled trials (RCTs) published as a full text and regarding nevirapine-based regimens used as a initial treatment for HIV infection. Meta-analysis was performed with RevMan(®) V 5.2 software.
Results: Twelve RCTs were included in the systematic review and all of them were suitable for meta-analysis. Results of the meta-analysis have shown that nevirapine, efavirenz, and ritonavir-boosted protease inhibitor, added to the background regimens, were equally effective in terms of reaching undetectable plasma HIV RNA level as well as risk of disease progression or death. Compared with ritonavir-boosted protease inhibitor-based regimens, nevirapine-based regimens statistically significantly increased the risk of discontinuation of assigned treatment (RR=3.10; 95% CI: 1.14-8.41; p<0.05).
Conclusions: Despite limited RCTs data available for particular comparisons, our results suggest that nevirapine-based regimens may be considered for first-line treatment of HIV-infected adults, due to their comparable efficacy to the other currently recommended initial antiretroviral therapies.
Conflict of interest statement
Figures
References
-
- Panel on Antiretroviral Guidelines for Adults and Adolescents Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents Department of Health and Human Services. Available: http://aidsinfo.nih.gov/ContentFiles/. AdultandAdolescentGL pdf Accessed March 17 (2013).
-
- FDA resources page; Food and Drug Administration Web site. Available: http://www.fda.gov/ForConsumers/ByAudience/ForPatientAdvocates/HIVandAID.... Accessed January 25, 2013.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical