Role of key salt bridges in thermostability of G. thermodenitrificans EstGtA2: distinctive patterns within the new bacterial lipolytic enzyme subfamily XIII.2 [corrected]
- PMID: 24116134
- PMCID: PMC3792869
- DOI: 10.1371/journal.pone.0076675
Role of key salt bridges in thermostability of G. thermodenitrificans EstGtA2: distinctive patterns within the new bacterial lipolytic enzyme subfamily XIII.2 [corrected]
Erratum in
-
Correction: Role of Key Salt Bridges in Thermostability of G. thermodenitrificans EstGtA2: Distinctive Patterns within the New Bacterial Lipolytic Enzyme Family XV.PLoS One. 2015 Aug 25;10(8):e0136940. doi: 10.1371/journal.pone.0136940. eCollection 2015. PLoS One. 2015. PMID: 26305892 Free PMC article. No abstract available.
Abstract
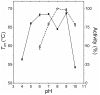
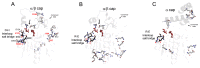
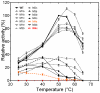
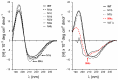
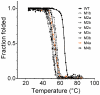
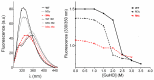
Bacterial lipolytic enzymes were originally classified into eight different families defined by Arpigny and Jaeger (families I-VIII). Recently, the discovery of new lipolytic enzymes allowed for extending the original classification to fourteen families (I-XIV). We previously reported that G. thermodenitrificans EstGtA2 (access no. AEN92268) belonged to a novel group of bacterial lipolytic enzymes. Here we propose a 15(th) family (family XV) and suggest criteria for the assignation of protein sequences to the N' subfamily. Five selected salt bridges, hallmarks of the N' subfamily (E3/R54, E12/R37, E66/R140, D124/K178 and D205/R220) were disrupted in EstGtA2 using a combinatorial alanine-scanning approach. A set of 14 (R/K→A) mutants was produced, including five single, three double, three triple and three quadruple mutants. Despite a high tolerance to non-conservative mutations for folding, all the alanine substitutions were destabilizing (decreasing T m by 5 to 14°C). A particular combination of four substitutions exceeded this tolerance and prevents the correct folding of EstGtA2, leading to enzyme inactivation. Although other mutants remain active at low temperatures, the accumulation of more than two mutations had a dramatic impact on EstGtA2 activity at high temperatures suggesting an important role of these conserved salt bridge-forming residues in thermostability of lipolytic enzymes from the N' subfamily. We also identified a particular interloop salt bridge in EstGtA2 (D194/H222), located at position i -2 and i -4 residues from the catalytic Asp and His respectively which is conserved in other related bacterial lipolytic enzymes (families IV and XIII) with high tolerance to mutations and charge reversal. We investigated the role of residue identity at position 222 in controlling stability-pH dependence in EstGtA2. The introduction of a His to Arg mutation led to increase thermostability under alkaline pH. Our results suggest primary targets for optimization of EstGtA2 for specific biotechnological purposes.
Conflict of interest statement
Figures
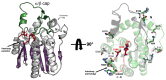





Similar articles
-
Impact of Salt Concentration and pH on Surface Charged Residues: Controlling Protein Association Pathways in Carboxylesterase EstGtA2.Protein Pept Lett. 2017;24(6):561-572. doi: 10.2174/0929866524666170404162730. Protein Pept Lett. 2017. PMID: 28393686
-
N-terminal purification tag alters thermal stability of the carboxylesterase EstGtA2 from G. thermodenitrificans by impairing reversibility of thermal unfolding.Protein Pept Lett. 2012 Mar;19(3):264-9. doi: 10.2174/092986612799363181. Protein Pept Lett. 2012. PMID: 21933135
-
Efficient monoacylglycerol synthesis by carboxylesterase EstGtA2 from Geobacillus thermodenitrificans in a solvent-free two-phase system.J Biosci Bioeng. 2022 Aug;134(2):89-94. doi: 10.1016/j.jbiosc.2022.05.004. Epub 2022 May 26. J Biosci Bioeng. 2022. PMID: 35644798
-
Improvement in thermostability of xylanase from Geobacillus thermodenitrificans C5 by site directed mutagenesis.Enzyme Microb Technol. 2018 Apr;111:38-47. doi: 10.1016/j.enzmictec.2018.01.004. Epub 2018 Jan 6. Enzyme Microb Technol. 2018. PMID: 29421035
-
Proteins from hyperthermophiles: stability and enzymatic catalysis close to the boiling point of water.Adv Biochem Eng Biotechnol. 1998;61:37-85. doi: 10.1007/BFb0102289. Adv Biochem Eng Biotechnol. 1998. PMID: 9670797 Review.
Cited by
-
Molecular, biochemical and kinetic analysis of a novel, thermostable lipase (LipSm) from Stenotrophomonas maltophilia Psi-1, the first member of a new bacterial lipase family (XVIII).J Biol Res (Thessalon). 2018 Feb 8;25:4. doi: 10.1186/s40709-018-0074-6. eCollection 2018 Dec. J Biol Res (Thessalon). 2018. PMID: 29456971 Free PMC article.
-
Protein thermal stability enhancement by designing salt bridges: a combined computational and experimental study.PLoS One. 2014 Nov 13;9(11):e112751. doi: 10.1371/journal.pone.0112751. eCollection 2014. PLoS One. 2014. PMID: 25393107 Free PMC article.
-
Production of 2-Hydroxyisobutyric Acid from Methanol by Methylobacterium extorquens AM1 Expressing (R)-3-Hydroxybutyryl Coenzyme A-Isomerizing Enzymes.Appl Environ Microbiol. 2017 Jan 17;83(3):e02622-16. doi: 10.1128/AEM.02622-16. Print 2017 Feb 1. Appl Environ Microbiol. 2017. PMID: 27836853 Free PMC article.
-
Characterization of a Novel Alkalophilic Lipase From Aneurinibacillus thermoaerophilus: Lid Heterogeneity and Assignment to Family I.5.Protein J. 2017 Dec;36(6):478-488. doi: 10.1007/s10930-017-9743-9. Protein J. 2017. PMID: 28975457
-
Harnessing the potential of chloroplast-derived expression elements for enhanced production of cellulases in Escherichia coli.PeerJ. 2025 Jan 31;13:e18616. doi: 10.7717/peerj.18616. eCollection 2025. PeerJ. 2025. PMID: 39902317 Free PMC article.
References
-
- Montoro-García S, Martínez-Martínez I, Navarro-Fernández J, Takami H, García-Carmona F et al. (2009) Characterization of a novel carboxylesterase from Geobacillus kaustophilus HTA426 shows the existence of a new carboxylesterase family. J Bacteriol 191(9): 3076-3085. doi:10.1128/JB.01060-08. PubMed: 19304850. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

