Variation in one residue associated with the metal ion-dependent adhesion site regulates αIIbβ3 integrin ligand binding affinity
- PMID: 24116162
- PMCID: PMC3792891
- DOI: 10.1371/journal.pone.0076793
Variation in one residue associated with the metal ion-dependent adhesion site regulates αIIbβ3 integrin ligand binding affinity
Abstract
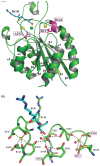
The Asp of the RGD motif of the ligand coordinates with the β I domain metal ion dependent adhesion site (MIDAS) divalent cation, emphasizing the importance of the MIDAS in ligand binding. There appears to be two distinct groups of integrins that differ in their ligand binding affinity and adhesion ability. These differences may be due to a specific residue associated with the MIDAS, particularly the β3 residue Ala(252) and corresponding Ala in the β1 integrin compared to the analogous Asp residue in the β2 and β7 integrins. Interestingly, mutations in the adjacent to MIDAS (ADMIDAS) of integrins α4β7 and αLβ2 increased the binding and adhesion abilities compared to the wild-type, while the same mutations in the α2β1, α5β1, αVβ3, and αIIbβ3 integrins demonstrated decreased ligand binding and adhesion. We introduced a mutation in the αIIbβ3 to convert this MIDAS associated Ala(252) to Asp. By combination of this mutant with mutations of one or two ADMIDAS residues, we studied the effects of this residue on ligand binding and adhesion. Then, we performed molecular dynamics simulations on the wild-type and mutant αIIbβ3 integrin β I domains, and investigated the dynamics of metal ion binding sites in different integrin-RGD complexes. We found that the tendency of calculated binding free energies was in excellent agreement with the experimental results, suggesting that the variation in this MIDAS associated residue accounts for the differences in ligand binding and adhesion among different integrins, and it accounts for the conflicting results of ADMIDAS mutations within different integrins. This study sheds more light on the role of the MIDAS associated residue pertaining to ligand binding and adhesion and suggests that this residue may play a pivotal role in integrin-mediated cell rolling and firm adhesion.
Conflict of interest statement
Figures

Similar articles
-
Mutagenesis studies of the β I domain metal ion binding sites on integrin αVβ3 ligand binding affinity.J Cell Biochem. 2012 Apr;113(4):1190-7. doi: 10.1002/jcb.23448. J Cell Biochem. 2012. PMID: 22095620
-
Regulation of integrin αIIbβ3 ligand binding and signaling by the metal ion binding sites in the β I domain.Biochemistry. 2011 Mar 29;50(12):2084-91. doi: 10.1021/bi2000092. Epub 2011 Feb 22. Biochemistry. 2011. PMID: 21309594
-
Ligand binding to integrin alphaIIbbeta3 is dependent on a MIDAS-like domain in the beta3 subunit.J Biol Chem. 1996 Sep 6;271(36):21978-84. doi: 10.1074/jbc.271.36.21978. J Biol Chem. 1996. PMID: 8703003
-
RGD, the Rho'd to cell spreading.Eur J Cell Biol. 2006 Apr;85(3-4):249-54. doi: 10.1016/j.ejcb.2005.08.003. Epub 2005 Sep 13. Eur J Cell Biol. 2006. PMID: 16546569 Review.
-
Integrin-ligand binding. Do integrins use a 'MIDAS touch' to grasp an Asp?Curr Biol. 1995 Jun 1;5(6):615-7. doi: 10.1016/s0960-9822(95)00124-2. Curr Biol. 1995. PMID: 7552170 Review.
Cited by
-
Application of Funnel Metadynamics to the Platelet Integrin αIIbβ3 in Complex with an RGD Peptide.Int J Mol Sci. 2024 Jun 14;25(12):6580. doi: 10.3390/ijms25126580. Int J Mol Sci. 2024. PMID: 38928286 Free PMC article.
-
Calculating toxic pressure for mixtures of endocrine disruptors.Heliyon. 2024 Jul 11;10(14):e34501. doi: 10.1016/j.heliyon.2024.e34501. eCollection 2024 Jul 30. Heliyon. 2024. PMID: 39149076 Free PMC article.
-
Nature-inspired surface modification strategies for implantable devices.Mater Today Bio. 2025 Feb 25;31:101615. doi: 10.1016/j.mtbio.2025.101615. eCollection 2025 Apr. Mater Today Bio. 2025. PMID: 40115053 Free PMC article. Review.
-
αIIbβ3: structure and function.J Thromb Haemost. 2015 Jun;13 Suppl 1(Suppl 1):S17-25. doi: 10.1111/jth.12915. J Thromb Haemost. 2015. PMID: 26149019 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

