The expression of CD90/Thy-1 in hepatocellular carcinoma: an in vivo and in vitro study
- PMID: 24116172
- PMCID: PMC3792890
- DOI: 10.1371/journal.pone.0076830
The expression of CD90/Thy-1 in hepatocellular carcinoma: an in vivo and in vitro study
Abstract
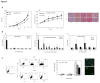
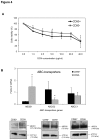
Although the CD90 (Thy-1) was proposed as biomarker of several tumors and cancer stem cells, the involvement of this molecule in the progression of hepatocellular carcinoma (HCC) and other less frequent hepatic neoplasms is still undefined. The distribution of CD90 was investigated both in in vivo (human tissues samples) and in vitro (human HCC cell line JHH-6). A total of 67 liver tumors were analyzed: 51 HCC, 6 cholangiocarcinoma and 10 hepatoblastoma. In all cases, paired tissue sample of both the tumor and cirrhotic liver was available. Hepatic tissue obtained in 12 healthy livers was used as control. CD90 gene expression was studied by RT-qPCR, protein expression was assessed by quantitative Western Blot, immunofluorescence and flow cytometry. The CD90 expression analysis showed a significant increment in tumor compared to both its paired cirrhotic tissue and normal liver (p<0.05 and p<0.001, respectively). This increase was accompanied by the up-regulation of stromal component in the cancer, as demonstrated by alpha smooth muscle actin staining. In vitro analysis of JHH-6 cell line showed a higher proliferation capacity of CD90(+) compared to CD90(-) cells (p<0.001), also noticed in 3D clonogenic assay (p<0.05), associated by a significant higher expression of the promoting factors (hepatocyte growth factor, fibroblast associated protein and alpha smooth muscle actin 2). A higher expression of the breast cancer resistance protein was found in CD90(+) subpopulation while the multidrug resistance protein 1 showed an opposite behavior. Collectively, these results point to the importance of CD90 in the HCC.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

