Lymphocytes and macrophages are infected by Theileria equi, but T cells and B cells are not required to establish infection in vivo
- PMID: 24116194
- PMCID: PMC3792048
- DOI: 10.1371/journal.pone.0076996
Lymphocytes and macrophages are infected by Theileria equi, but T cells and B cells are not required to establish infection in vivo
Abstract
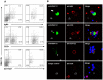
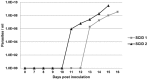
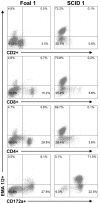
Theileria equi has a biphasic life cycle in horses, with a period of intraleukocyte development followed by patent erythrocytic parasitemia that causes acute and sometimes fatal hemolytic disease. Unlike Theileria spp. that infect cattle (Theileria parva and Theileria annulata), the intraleukocyte stage (schizont) of Theileria equi does not cause uncontrolled host cell proliferation or other significant pathology. Nevertheless, schizont-infected leukocytes are of interest because of their potential to alter host cell function and because immune responses directed against this stage could halt infection and prevent disease. Based on cellular morphology, Theileria equi has been reported to infect lymphocytes in vivo and in vitro, but the specific phenotype of schizont-infected cells has yet to be defined. To resolve this knowledge gap in Theileria equi pathogenesis, peripheral blood mononuclear cells were infected in vitro and the phenotype of infected cells determined using flow cytometry and immunofluorescence microscopy. These experiments demonstrated that the host cell range of Theileria equi was broader than initially reported and included B lymphocytes, T lymphocytes and monocyte/macrophages. To determine if B and T lymphocytes were required to establish infection in vivo, horses affected with severe combined immunodeficiency (SCID), which lack functional B and T lymphocytes, were inoculated with Theileria equi sporozoites. SCID horses developed patent erythrocytic parasitemia, indicating that B and T lymphocytes are not necessary to complete the Theileria equi life cycle in vivo. These findings suggest that the factors mediating Theileria equi leukocyte invasion and intracytoplasmic differentiation are common to several leukocyte subsets and are less restricted than for Theileria annulata and Theileria parva. These data will greatly facilitate future investigation into the relationships between Theileria equi leukocyte tropism and pathogenesis, breed susceptibility, and strain virulence.
Conflict of interest statement
Figures


Similar articles
-
Infectivity and cross-immunity studies of Theileria lestoquardi and Theileria annulata in sheep and cattle: II. In vitro studies.Vet Parasitol. 1999 Apr 12;82(3):193-204. doi: 10.1016/s0304-4017(99)00014-x. Vet Parasitol. 1999. PMID: 10348098
-
Infection of bovine monocyte/macrophage populations with Theileria annulata and Theileria parva.Vet Immunol Immunopathol. 1989 Nov 15;22(4):355-68. doi: 10.1016/0165-2427(89)90171-2. Vet Immunol Immunopathol. 1989. PMID: 2516673
-
Serum-free in vitro cultivation of Theileria annulata and Theileria parva schizont-infected lymphocytes.Transbound Emerg Dis. 2020 Mar;67 Suppl 1:35-39. doi: 10.1111/tbed.13348. Transbound Emerg Dis. 2020. PMID: 32174041
-
Innate and adaptive immune responses co-operate to protect cattle against Theileria annulata.Parasitol Today. 1999 Jul;15(7):268-74. doi: 10.1016/s0169-4758(99)01466-0. Parasitol Today. 1999. PMID: 10377528 Review.
-
Immunity and vaccine development in the bovine theilerioses.Adv Parasitol. 1999;44:41-97. doi: 10.1016/s0065-308x(08)60230-4. Adv Parasitol. 1999. PMID: 10563395 Review.
Cited by
-
The Complexity of Piroplasms Life Cycles.Front Cell Infect Microbiol. 2018 Jul 23;8:248. doi: 10.3389/fcimb.2018.00248. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 30083518 Free PMC article. Review.
-
Characterization of the Rhipicephalus (Boophilus) microplus Sialotranscriptome Profile in Response to Theileria equi Infection.Pathogens. 2021 Feb 4;10(2):167. doi: 10.3390/pathogens10020167. Pathogens. 2021. PMID: 33557100 Free PMC article.
-
Expression of IL-10 and TGF-β1 in horses experimentally infected with T. equi merozoites is associated with antibody production but not modulation of pro-inflammatory responses.Front Immunol. 2024 May 13;15:1370255. doi: 10.3389/fimmu.2024.1370255. eCollection 2024. Front Immunol. 2024. PMID: 38803499 Free PMC article.
-
Theileria equi isolates vary in susceptibility to imidocarb dipropionate but demonstrate uniform in vitro susceptibility to a bumped kinase inhibitor.Parasit Vectors. 2015 Jan 20;8:33. doi: 10.1186/s13071-014-0611-6. Parasit Vectors. 2015. PMID: 25600252 Free PMC article.
-
Twenty Years of Equine Piroplasmosis Research: Global Distribution, Molecular Diagnosis, and Phylogeny.Pathogens. 2020 Nov 8;9(11):926. doi: 10.3390/pathogens9110926. Pathogens. 2020. PMID: 33171698 Free PMC article. Review.
References
-
- Schein E (1988) Equine Babesiosis. In: Ristic M, Babesiosis of Domestic Animals and Man. Boca Raton: CRC Press. 197–208.
-
- De Waal DT (1992) Equine piroplasmosis: a review. Br Vet J 148: 6–14. - PubMed
-
- Mehlhorn H, Schein E (1998) Redescription of Babesia equi Laveran, 1901 as Theileria equi Mehlhorn, Schein 1998. Parasitol Res 84: 467–475. - PubMed
-
- Schein E, Rehbein G, Voigt WP, Zweygarth E (1981) Babesia equi (Laveran 1901) 1. Development in horses and in lymphocyte culture. Tropenmed Parasitol 32: 223–227. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

