An anti-CD34 antibody-functionalized clinical-grade POSS-PCU nanocomposite polymer for cardiovascular stent coating applications: a preliminary assessment of endothelial progenitor cell capture and hemocompatibility
- PMID: 24116210
- PMCID: PMC3793009
- DOI: 10.1371/journal.pone.0077112
An anti-CD34 antibody-functionalized clinical-grade POSS-PCU nanocomposite polymer for cardiovascular stent coating applications: a preliminary assessment of endothelial progenitor cell capture and hemocompatibility
Abstract
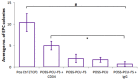
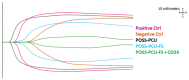
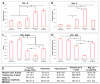
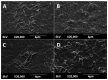
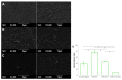
In situ endothelialization of cardiovascular implants has emerged in recent years as an attractive means of targeting the persistent problems of thrombosis and intimal hyperplasia. This study aimed to investigate the efficacy of immobilizing anti-CD34 antibodies onto a POSS-PCU nanocomposite polymer surface to sequester endothelial progenitor cells (EPCs) from human blood, and to characterize the surface properties and hemocompatibility of this surface. Amine-functionalized fumed silica was used to covalently conjugate anti-CD34 to the polymer surface. Water contact angle, fluorescence microscopy, and scanning electron microscopy were used for surface characterization. Peripheral blood mononuclear cells (PBMCs) were seeded on modified and pristine POSS-PCU polymer films. After 7 days, adhered cells were immunostained for the expression of EPC and endothelial cell markers, and assessed for the formation of EPC colonies. Hemocompatibility was assessed by thromboelastography, and platelet activation and adhesion assays. The number of EPC colonies formed on anti-CD34-coated POSS-PCU surfaces was not significantly higher than that of POSS-PCU (5.0±1.0 vs. 1.7±0.6, p>0.05). However, antibody conjugation significantly improved hemocompatibility, as seen from the prolonged reaction and clotting times, decreased angle and maximum amplitude (p<0.05), as well as decreased platelet adhesion (76.8±7.8 vs. 8.4±0.7, p<0.05) and activation. Here, we demonstrate that POSS-PCU surface immobilized anti-CD34 antibodies selectively captured CD34+ cells from peripheral blood, although only a minority of these were EPCs. Nevertheless, antibody conjugation significantly improves the hemocompatibility of POSS-PCU, and should therefore continue to be explored in combination with other strategies to improve the specificity of EPC capture to promote in situ endothelialization.
Conflict of interest statement
Figures








References
-
- Boudriot E, Thiele H, Walther T, Liebetrau C, Boeckstegers P et al. (2011) Randomized comparison of percutaneous coronary intervention with sirolimus-eluting stents versus coronary artery bypass grafting in unprotected left main stem stenosis. J Am Coll Cardiol 57: 538-545. doi:10.1016/S0735-1097(11)60538-X. PubMed: 21272743. - DOI - PubMed
-
- Mohr FW, Morice M-C, Kappetein AP, Feldman TE, Ståhle E et al. (2013) Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet 381: 629-638. doi:10.1016/S0140-6736(13)60141-5. PubMed: 23439102. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

