Regional fibronectin and collagen fibril co-assembly directs cell proliferation and microtissue morphology
- PMID: 24116223
- PMCID: PMC3792918
- DOI: 10.1371/journal.pone.0077316
Regional fibronectin and collagen fibril co-assembly directs cell proliferation and microtissue morphology
Abstract
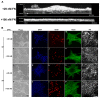
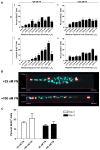
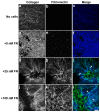
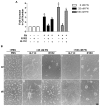
The extracellular matrix protein, fibronectin stimulates cells to self-assemble into three-dimensional multicellular structures by a mechanism that requires the cell-dependent conversion of soluble fibronectin molecules into insoluble fibrils. Fibronectin also binds to collagen type I and mediates the co-assembly of collagen fibrils into the extracellular matrix. Here, the role of collagen-fibronectin binding in fibronectin-induced cellular self-assembly was investigated using fibronectin-null fibroblasts in an in vitro model of tissue formation. High resolution, two-photon immunofluorescence microscopy was combined with second harmonic generation imaging to examine spatial and temporal relationships among fibronectin and collagen fibrils, actin organization, cell proliferation, and microtissue morphology. Time course studies coupled with simultaneous 4-channel multiphoton imaging identified regional differences in fibronectin fibril conformation, collagen fibril remodeling, actin organization, and cell proliferation during three-dimensional cellular self-assembly. Regional differences in cell proliferation and fibronectin structure were dependent on both soluble fibronectin concentration and fibronectin-collagen interactions. Fibronectin-collagen binding was not necessary for either fibronectin matrix formation or intercellular cohesion. However, inhibiting fibronectin binding to collagen reduced collagen fibril remodeling, decreased fibronectin fibril extension, blocked fibronectin-induced cell proliferation, and altered microtissue morphology. Furthermore, continual fibronectin-collagen binding was necessary to maintain both cell proliferation and microtissue morphology. Collectively, these data suggest that the complex changes in extracellular matrix and cytoskeletal remodeling that mediate tissue assembly are driven, in part, by regional variations in cell-mediated fibronectin-collagen co-assembly.
Conflict of interest statement
Figures





Similar articles
-
Cooperative effects of fibronectin matrix assembly and initial cell-substrate adhesion strength in cellular self-assembly.Acta Biomater. 2016 Mar 1;32:198-209. doi: 10.1016/j.actbio.2015.12.032. Epub 2015 Dec 19. Acta Biomater. 2016. PMID: 26712598 Free PMC article.
-
Opposing effects of collagen I and vitronectin on fibronectin fibril structure and function.Matrix Biol. 2014 Feb;34:33-45. doi: 10.1016/j.matbio.2014.01.017. Epub 2014 Feb 6. Matrix Biol. 2014. PMID: 24509439 Free PMC article.
-
Mechanical properties of the extracellular matrix influence fibronectin fibril assembly in vitro.Exp Cell Res. 1995 Mar;217(1):109-17. doi: 10.1006/excr.1995.1069. Exp Cell Res. 1995. PMID: 7867709
-
Assembly of fibronectin extracellular matrix.Annu Rev Cell Dev Biol. 2010;26:397-419. doi: 10.1146/annurev-cellbio-100109-104020. Annu Rev Cell Dev Biol. 2010. PMID: 20690820 Free PMC article. Review.
-
Fibronectin fibrillogenesis, a cell-mediated matrix assembly process.Matrix Biol. 2005 Sep;24(6):389-99. doi: 10.1016/j.matbio.2005.06.008. Matrix Biol. 2005. PMID: 16061370 Review.
Cited by
-
Long term morphological characterization of mesenchymal stromal cells 3D spheroids built with a rapid method based on entry-level equipment.Cytotechnology. 2016 Dec;68(6):2479-2490. doi: 10.1007/s10616-016-9969-y. Epub 2016 Mar 29. Cytotechnology. 2016. PMID: 27023795 Free PMC article.
-
Collagen inhibitory peptide R1R2 mediates vascular remodeling by decreasing inflammation and smooth muscle cell activation.PLoS One. 2015 Feb 12;10(2):e0117356. doi: 10.1371/journal.pone.0117356. eCollection 2015. PLoS One. 2015. PMID: 25675397 Free PMC article.
-
The Structural Interactions of Molecular and Fibrillar Collagen Type I with Fibronectin and Its Role in the Regulation of Mesenchymal Stem Cell Morphology and Functional Activity.Int J Mol Sci. 2022 Oct 20;23(20):12577. doi: 10.3390/ijms232012577. Int J Mol Sci. 2022. PMID: 36293432 Free PMC article.
-
Recombinant Extracellular Matrix Protein Fragments Support Human Embryonic Stem Cell Chondrogenesis.Tissue Eng Part A. 2018 Jun;24(11-12):968-978. doi: 10.1089/ten.TEA.2017.0285. Epub 2018 Feb 7. Tissue Eng Part A. 2018. PMID: 29279011 Free PMC article.
-
Exogenous supply of Hsp47 triggers fibrillar collagen deposition in skin cell cultures in vitro.BMC Mol Cell Biol. 2020 Mar 30;21(1):22. doi: 10.1186/s12860-020-00267-0. BMC Mol Cell Biol. 2020. PMID: 32228452 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

