(18)F-FDG imaging of human atherosclerotic carotid plaques reflects gene expression of the key hypoxia marker HIF-1α
- PMID: 24116346
- PMCID: PMC3784801
(18)F-FDG imaging of human atherosclerotic carotid plaques reflects gene expression of the key hypoxia marker HIF-1α
Abstract
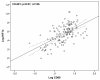
To investigate the association between gene expression of key molecular markers of hypoxia and inflammation in atherosclerotic carotid lesions with 2-deoxy-2-[(18)F]fluoro-D-glucose ((18)F-FDG) uptake as determined clinically by positron emission tomography (PET). Studies using PET have demonstrated (18)F-FDG-uptake in patients with confirmed plaques of the carotid artery. Inflammatory active or "vulnerable" plaques progressively increase in bulk, develop necrotic cores, poor vessel-wall vascularization and become prone to hypoxia. We used quantitative polymerase-chain reaction (qPCR) to determine gene expression of hypoxia-inducible factor 1α (HIF-1α) and cluster of differentiation 68 (CD68) on plaques recovered by carotid endarterectomy (CEA) in 18 patients. Gene expression was compared with (18)F-FDG-uptake quantified as the maximum standardized uptake value (SUVmax) on co-registered PET/computed tomography (CT) scans performed the day before CEA. Immunohistochemistry was used to validate target-gene protein expression. In univariate linear regression analysis HIF-1α was significantly correlated with (18)F-FDG-uptake (SUVmax) as was CD68. A two-tailed Pearson regression model demonstrated that HIF-1α and CD68 gene expression co-variated and accordingly when entering the variables into multivariate linear regression models with SUV-values as dependent variables, HIF-1α was eliminated in the final models. (18)F-FDG-uptake (SUVmax) is correlated with HIF-1α gene expression indicating an association between hypoxia and glucose metabolism in vivo. The marker of inflammation CD68 is also associated with (18)F-FDG-uptake (SUVmax). As CD68 and HIF-1α gene expression co-variate their information is overlapping.
Keywords: 18F-FDG PET/CT imaging; HIF-1α; Hypoxia; carotid atherosclerosis; gene expression.
Figures




References
-
- Hobson RW, Mackey WC, Ascher E, Murad MH, Calligaro KD, Comerota AJ, Montori VM, Eskandari MK, Massop DW, Bush RL, Lal BK, Perler BA. Management of atherosclerotic carotid artery disease: clinical practice guidelines of the Society for Vascular Surgery. J Vasc Surg. 2008;48:480–486. - PubMed
-
- Rerkasem K, Rothwell PM. Carotid endarterectomy for symptomatic carotid stenosis. Cochrane Database Syst Rev. 2011:CD001081. - PubMed
-
- Brott TG, Hobson RW, Howard G, Roubin GS, Clark WM, Brooks W, Mackey A, Hill MD, Leimgruber PP, Sheffet AJ, Howard VJ, Moore WS, Voeks JH, Hopkins LN, Cutlip DE, Cohen DJ, Popma JJ, Ferguson RD, Cohen SN, Blackshear JL, Silver FL, Mohr JP, Lal BK, Meschia JF. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010;363:11–23. - PMC - PubMed
-
- Keitzer WF, Lichti EL, DeWeese MS. Clinical evaluation and correction of carotid artery occlusive disease. Use of the Doppler ultrasonic flowmeter. Am J Surg. 1972;124:697–700. - PubMed
-
- Chaturvedi S, Bruno A, Feasby T, Holloway R, Benavente O, Cohen SN, Cote R, Hess D, Saver J, Spence JD, Stern B, Wilterdink J. Carotid endarterectomy--an evidence-based review: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2005;65:794–801. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
