Bupivacaine-induced cellular entry of QX-314 and its contribution to differential nerve block
- PMID: 24117225
- PMCID: PMC3904263
- DOI: 10.1111/bph.12466
Bupivacaine-induced cellular entry of QX-314 and its contribution to differential nerve block
Abstract
Background and purpose: Selective nociceptor fibre block is achieved by introducing the cell membrane impermeant sodium channel blocker lidocaine N-ethyl bromide (QX-314) through transient receptor potential V1 (TRPV1) channels into nociceptors. We screened local anaesthetics for their capacity to activate TRP channels, and characterized the nerve block obtained by combination with QX-314.
Experimental approach: We investigated TRP channel activation in dorsal root ganglion (DRG) neurons by calcium imaging and patch-clamp recordings, and cellular QX-314 uptake by MS. To characterize nerve block, compound action potential (CAP) recordings from isolated nerves and behavioural responses were analysed.
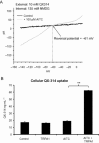
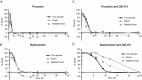
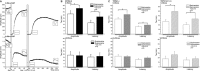
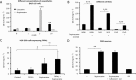
Key results: Of the 12 compounds tested, bupivacaine was the most potent activator of ruthenium red-sensitive calcium entry in DRG neurons and activated heterologously expressed TRPA1 channels. QX-314 permeated through TRPA1 channels and accumulated intracellularly after activation of these channels. Upon sciatic injections, QX-314 markedly prolonged bupivacaine's nociceptive block and also extended (to a lesser degree) its motor block. Bupivacaine's blockade of C-, but not A-fibre, CAPs in sciatic nerves was extended by co-application of QX-314. Surprisingly, however, this action was the same in wild-type, TRPA1-knockout and TRPV1/TRPA1-double knockout mice, suggesting a TRP-channel independent entry pathway. Consistent with this, high doses of bupivacaine promoted a non-selective, cellular uptake of QX-314.
Conclusions and implications: Bupivacaine, combined with QX-314, produced a long-lasting sensory nerve block. This did not require QX-314 permeation through TRPA1, although bupivacaine activated these channels. Regardless of entry pathway, the greatly extended duration of block produced by QX-314 and bupivacaine may be clinically useful.
Keywords: CAP; DRG; QX-314; TRPA1; TRPV1; nociception; sciatic nerve.
© 2013 The British Pharmacological Society.
Figures




References
-
- Banke TG, Chaplan SR, Wickenden AD. Dynamic changes in the TRPA1 selectivity filter lead to progressive but reversible pore dilation. Am J Physiol Cell Physiol. 2010;298:C1457–C1468. - PubMed
-
- Banke TG, Wickenden AD. Intracellular zinc irritates TRPA1. Nat Chem Biol. 2009;5:141–142. - PubMed
-
- Binshtok AM, Bean BP, Woolf CJ. Inhibition of nociceptors by TRPV1-mediated entry of impermeant sodium channel blockers. Nature. 2007;449:607–610. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

