Peptide pheromone signaling in Streptococcus and Enterococcus
- PMID: 24118108
- PMCID: PMC4103628
- DOI: 10.1111/1574-6976.12046
Peptide pheromone signaling in Streptococcus and Enterococcus
Abstract
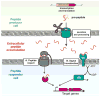
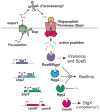
Intercellular chemical signaling in bacteria, commonly referred to as quorum sensing (QS), relies on the production and detection of compounds known as pheromones to elicit coordinated responses among members of a community. Pheromones produced by Gram-positive bacteria are comprised of small peptides. Based on both peptide structure and sensory system architectures, Gram-positive bacterial signaling pathways may be classified into one of four groups with a defining hallmark: cyclical peptides of the Agr type, peptides that contain Gly-Gly processing motifs, sensory systems of the RNPP family, or the recently characterized Rgg-like regulatory family. The recent discovery that Rgg family members respond to peptide pheromones increases substantially the number of species in which QS is likely a key regulatory component. These pathways control a variety of fundamental behaviors including conjugation, natural competence for transformation, biofilm development, and virulence factor regulation. Overlapping QS pathways found in multiple species and pathways that utilize conserved peptide pheromones provide opportunities for interspecies communication. Here we review pheromone signaling identified in the genera Enterococcus and Streptococcus, providing examples of all four types of pathways.
Keywords: Firmicutes; biofilms; competence; gene regulation; intercellular communication; virulence.
© 2013 Federation of European Microbiological Societies. Published by John Wiley & Sons Ltd. All rights reserved.
Figures




References
-
- Aspiras MB, Ellen RP, Cvitkovitch DG. ComX activity of Streptococcus mutans growing in biofilms. FEMS Microbiol Lett. 2004;238:167–174. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

