Characterization of different regimens for initiating anagrelide in patients with essential thrombocythemia who are intolerant or refractory to their current cytoreductive therapy: results from the multicenter FOX study of 177 patients in France
- PMID: 24118452
- PMCID: PMC4232889
- DOI: 10.1111/ejh.12210
Characterization of different regimens for initiating anagrelide in patients with essential thrombocythemia who are intolerant or refractory to their current cytoreductive therapy: results from the multicenter FOX study of 177 patients in France
Abstract
Objectives: To identify switch modalities used when initiating second- or third-line anagrelide for essential thrombocythemia (ET), assess whether anagrelide is initiated consistently with Summary of Product Characteristics (SPC) recommendations, and determine whether different observed switch regimens have any relationship with maintenance, platelet response, or tolerability.
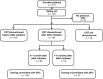
Methods: This observational study was conducted across 43 centers in France. High-risk patients (>60 yr of age and/or history of thrombosis and/or platelet count >1000 × 10(9) /L) with ET starting second- or third-line anagrelide therapy were identified and monitored for 6 months.
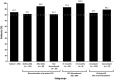
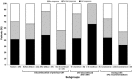
Results: A total of 177 patients were enrolled. The SPC-recommended starting dose (1 mg/d) was used in 52.6% of patients; 0.5 mg/d was used in 41.1%. 77.1% of patients underwent an anagrelide dose increase during the study. At 6-month follow-up, 84.7% of patients (n = 144/170) were still receiving anagrelide; 70.6% (n = 120/170) achieved a platelet response. A higher proportion of patients who discontinued previous cytoreductive therapy (CRT) after initiating anagrelide achieved a platelet response (n = 34/39, 87.2%) vs. patients who discontinued their previous CRT before anagrelide initiation (n = 77/115, 67.0%). Platelet response rates were higher in patients whose anagrelide initiation was consistent (n = 100/133, 75.2%) vs. inconsistent (n = 20/37, 54.1%) with the SPC. The incidence of adverse drug reactions was lower in patients whose anagrelide treatment was consistent (n = 52/133, 39.1%) vs. inconsistent (n = 25/37, 67.6%) with the SPC.
Conclusions: To our knowledge, the FOX study provides the first comprehensive real-world data on the modalities used when switching from previous CRT to anagrelide. Highest platelet responses were observed when previous CRT was discontinued after anagrelide initiation or when anagrelide was initiated consistently with the SPC. Safety data corresponded with the SPC.
Trial registration: ClinicalTrials.gov NCT01192347.
Keywords: anagrelide; blood platelets; essential thrombocythemia; hydroxycarbamide; intolerance; myeloproliferative disorders; platelet count; resistance; switch.
© 2013 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Figures
References
-
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW. WHO Classification of Tumours of Haemopoietic and Lymphoid Tissues. 4th edn. Lyon: IARC Press; 2008.
-
- Johansson P. Epidemiology of the myeloproliferative disorders polycythemia vera and essential thrombocythemia. Semin Thromb Hemost. 2006;32:171–3. - PubMed
-
- Tefferi A, Vainchenker W. Myeloproliferative neoplasms: molecular pathophysiology, essential clinical understanding, and treatment strategies. J Clin Oncol. 2011;29:573–82. - PubMed
-
- European Medicines Agency. Xagrid Summary of Product Characteristics. Last update 2013. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Info.... Accessed May 7, 2013.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

