Recombinant DNA production of spider silk proteins
- PMID: 24119078
- PMCID: PMC3815454
- DOI: 10.1111/1751-7915.12081
Recombinant DNA production of spider silk proteins
Abstract
Spider dragline silk is considered to be the toughest biopolymer on Earth due to an extraordinary combination of strength and elasticity. Moreover, silks are biocompatible and biodegradable protein-based materials. Recent advances in genetic engineering make it possible to produce recombinant silks in heterologous hosts, opening up opportunities for large-scale production of recombinant silks for various biomedical and material science applications. We review the current strategies to produce recombinant spider silks.
© 2013 The Authors. Microbial Biotechnology published by John Wiley & Sons Ltd and Society for Applied Microbiology.
Figures


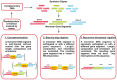
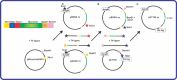

References
-
- Asakura T, Umemura K, Nakazawa Y, Hirose H, Higham J, Knight D. Some observations on the structure and function of the spinning apparatus in the silkworm Bombyx mori. Biomacromolecules. 2007;8:175–181. - PubMed
-
- Barr LA, Fahnestock SR, Yang J. Production and purification of recombinant DP1B silk -like protein in plants. Mol Breed. 2004;13:345–356.
-
- Brooks AE, Steinkraus HB, Nelson SR, Lewis RV. An investigation of the divergence of major ampullate silk fibers from Nephila clavipes and Argiope aurantia. Biomacromolecules. 2005;6:3095–3099. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

