Plasticity of primary microglia on micropatterned geometries and spontaneous long-distance migration in microfluidic channels
- PMID: 24119251
- PMCID: PMC3853476
- DOI: 10.1186/1471-2202-14-121
Plasticity of primary microglia on micropatterned geometries and spontaneous long-distance migration in microfluidic channels
Abstract
Background: Microglia possess an elevated grade of plasticity, undergoing several structural changes based on their location and state of activation. The first step towards the comprehension of microglia's biology and functional responses to an extremely mutable extracellular milieu, consists in discriminating the morphological features acquired by cells maintained in vitro under diverse environmental conditions. Previous work described neither primary microglia grown on artificially patterned environments which impose physical cues and constraints, nor long distance migration of microglia in vitro. To this aim, the present work exploits artificial bio-mimetic microstructured substrates with pillar-shaped or line-grating geometries fabricated on poly(dimethylsiloxane) by soft lithography, in addition to microfluidic devices, and highlights some morphological/functional characteristics of microglia which were underestimated or unknown so far.
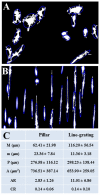
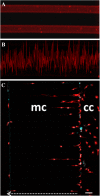
Results: We report that primary microglia selectively adapt to diverse microstructured substrates modifying accordingly their morphological features and behavior. On micropatterned pillar-shaped geometries, microglia appear multipolar, extend several protrusions in all directions and form distinct pseudopodia. On both micropatterned line-grating geometries and microfluidic channels, microglia extend the cytoplasm from a roundish to a stretched, flattened morphology and assume a filopodia-bearing bipolar structure. Finally, we show that in the absence of any applied chemical gradient, primary microglia spontaneously moves through microfluidic channels for a distance of up to 500 μm in approximately 12 hours, with an average speed of 0.66 μm/min.
Conclusions: We demonstrate an elevated grade of microglia plasticity in response to a mutable extracellular environment, thus making these cells an appealing population to be further exploited for lab on chip technologies. The development of microglia-based microstructured substrates opens the road to novel hybrid platforms for testing drugs for neuroinflammatory diseases.
Figures






References
-
- del Rio-Hortega P. In: Cytology and Cellular Pathology of the Nervous System. Penfield W, editor. New York: Hoeber; 1932. Microglia; pp. 482–534.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

