Camel milk ameliorates steatohepatitis, insulin resistance and lipid peroxidation in experimental non-alcoholic fatty liver disease
- PMID: 24119413
- PMCID: PMC3852981
- DOI: 10.1186/1472-6882-13-264
Camel milk ameliorates steatohepatitis, insulin resistance and lipid peroxidation in experimental non-alcoholic fatty liver disease
Abstract
Background: Camel milk (CM) is gaining increasing recognition due to its beneficial effects in the control and prevention of multiple health problems. The current study aimed to investigate the effects of CM on the hepatic biochemical and cellular alterations induced by a high-fat, cholesterol-rich diet (HCD), specifically, non-alcoholic fatty liver disease (NAFLD).
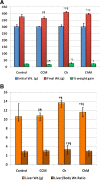
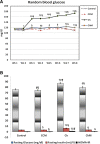
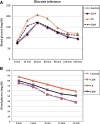
Methods: Seventy male Wistar rats were divided into four groups: the Control (C) Group fed a standard diet; the Control + camel milk (CCM) Group fed a standard diet and CM, the Cholesterol (Ch) Group fed a HCD with no CM, and the Cholesterol + camel milk (ChM) Group fed a HCD and CM. The following parameters were investigated in the studied groups; basal, weekly random and final fasting blood glucose levels, intraperitoneal glucose tolerance test (GTT) and insulin tolerance test (ITT), serum insulin, serum lipids, liver functions, lipid peroxidation products, the antioxidant activity of catalase (CAT) and the levels of reduced glutathione (GSH). In addition, HOMA-IR as an index of insulin resistance (IR) and the histopathology of the hepatic tissue were assessed.
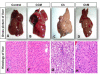
Results: The Ch Group developed features similar to those of non-alcoholic steatohepatitis (NASH), characterized by hepatic steatosis; inflammatory cellular infiltration in liver tissue; altered liver functions; and increased total cholesterol, triglycerides, low-density lipoprotein cholesterol, very-low-density lipoprotein cholesterol, atherogenic index (AI), blood glucose, IR, and malondialdehyde (MDA) levels. Additionally, feeding the HCD to animals in the Ch Group decreased CAT activity and the GSH and high-density lipoprotein (HDL) cholesterol levels. Camel milk intake for eight weeks decreased hepatic fat accumulation and inflammatory cellular infiltration, preserved liver function, increased the GSH levels and CAT activity, decreased the MDA levels, and ameliorated the changes in the lipid profile, AI, and IR in animals from the ChM Group.
Conclusions: CM has a unique composition that is rich in minerals; vitamins, insulin and insulin-like protein, and it increased HDL-cholesterol and ameliorated the biochemical and cellular features of NAFLD in rats that received a HCD. The antioxidant effect of CM is a likely mechanism for the altered metabolism and absorption of HCD in the presence of CM. Regular consumption of CM could provide a natural way to protect against NAFLD induced by a high-fat diet.
Figures

Similar articles
-
Camel milk ameliorates hyperglycaemia and oxidative damage in type-1 diabetic experimental rats.J Dairy Res. 2016 Aug;83(3):412-9. doi: 10.1017/S002202991600042X. J Dairy Res. 2016. PMID: 27600979
-
Preventive effect of curcumin on inflammation, oxidative stress and insulin resistance in high-fat fed obese rats.J Complement Integr Med. 2016 Jun 1;13(2):137-43. doi: 10.1515/jcim-2015-0070. J Complement Integr Med. 2016. PMID: 26845728
-
Levocetirizine ameliorates high fructose diet-induced insulin resistance, vascular dysfunction and hepatic steatosis in rats.Eur J Pharmacol. 2014 Oct 5;740:353-63. doi: 10.1016/j.ejphar.2014.07.021. Epub 2014 Jul 24. Eur J Pharmacol. 2014. PMID: 25064340
-
The Effect of Low Glycemic Index and Glycemic Load Diets on Hepatic Fat Mass, Insulin Resistance, and Blood Lipid Panels in Individuals with Nonalcoholic Fatty Liver Disease.Metab Syndr Relat Disord. 2019 Oct;17(8):389-396. doi: 10.1089/met.2019.0038. Epub 2019 Jul 15. Metab Syndr Relat Disord. 2019. PMID: 31305201 Review.
-
Increased very low density lipoprotein (VLDL) secretion, hepatic steatosis, and insulin resistance.Trends Endocrinol Metab. 2011 Sep;22(9):353-63. doi: 10.1016/j.tem.2011.04.007. Epub 2011 May 26. Trends Endocrinol Metab. 2011. PMID: 21616678 Free PMC article. Review.
Cited by
-
Camel Milk Mitigates Cyclosporine-Induced Renal Damage in Rats: Targeting p38/ERK/JNK MAPKs, NF-κB, and Matrix Metalloproteinases.Biology (Basel). 2021 May 17;10(5):442. doi: 10.3390/biology10050442. Biology (Basel). 2021. PMID: 34067576 Free PMC article.
-
Effect of camel milk on lipid profile among patients with diabetes: a systematic review, meta-analysis, and meta-regression of randomized controlled trials.BMC Complement Med Ther. 2023 Dec 4;23(1):438. doi: 10.1186/s12906-023-04257-5. BMC Complement Med Ther. 2023. PMID: 38049802 Free PMC article.
-
Anti-Oxidative and Immuno-Protective Effect of Camel Milk on Radiation-Induced Intestinal Injury in C57BL/6 J Mice.Dose Response. 2021 Mar 30;19(1):15593258211003798. doi: 10.1177/15593258211003798. eCollection 2021 Jan-Mar. Dose Response. 2021. PMID: 33867894 Free PMC article.
-
Anticancer Activity of Camel Milk via Induction of Autophagic Death in Human Colorectal and Breast Cancer Cells.Asian Pac J Cancer Prev. 2018 Dec 25;19(12):3501-3509. doi: 10.31557/APJCP.2018.19.12.3501. Asian Pac J Cancer Prev. 2018. PMID: 30583676 Free PMC article.
-
Non-Bovine Milk as Functional Foods with Focus on Their Antioxidant and Anti-Inflammatory Bioactivities.Antioxidants (Basel). 2025 Jun 27;14(7):801. doi: 10.3390/antiox14070801. Antioxidants (Basel). 2025. PMID: 40722905 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

