Immune synapse: conductor of orchestrated organelle movement
- PMID: 24119664
- PMCID: PMC4347664
- DOI: 10.1016/j.tcb.2013.09.005
Immune synapse: conductor of orchestrated organelle movement
Abstract
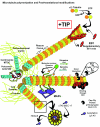
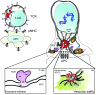
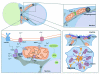
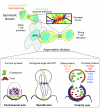
To ensure proper cell function, intracellular organelles are not randomly distributed within the cell, but polarized and highly constrained by the cytoskeleton and associated adaptor proteins. This relationship between distribution and function was originally found in neurons and epithelial cells; however, recent evidence suggests that it is a general phenomenon occurring in many highly specialized cells including T lymphocytes. Recent studies reveal that the orchestrated redistribution of organelles is dependent on antigen-specific activation of and immune synapse (IS) formation by T cells. This review highlights the functional implications of organelle polarization in early T cell activation and examines recent findings on how the IS sets the rhythm of organelle motion and the spread of the activation signal to the nucleus.
Keywords: immune synapse; microcluster; microtubule; mitochondria; signalosome; vesicle.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures

References
-
- Lasserre R, Alcover A. Cytoskeletal cross-talk in the control of T cell antigen receptor signaling. FEBS Lett. 2010;584:4845–4850. - PubMed
-
- Shaw AS, Filbert EL. Scaffold proteins and immune-cell signalling. Nat Rev Immunol. 2009;9:47–56. - PubMed
-
- Yanez-Mo M, et al. Tetraspanin-enriched microdomains: a functional unit in cell plasma membranes. Trends Cell Biol. 2009;19:434–446. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

